Clinical research in the community
- PMID: 38066876
- PMCID: PMC10727107
- DOI: 10.1182/hematology.2023000432
Clinical research in the community
Abstract
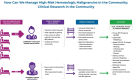
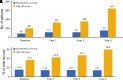
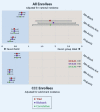
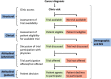
Most patients with high-risk hematologic malignancies are treated in community oncology practices near their residence. This is partly due to patients' ardent desire to be closer to home and trust in local caregivers. Treatments are increasingly complex, even as initial therapy, and more so upon relapse. Improved outcomes in the past decade are largely available through clinical trials primarily offered through academic medical centers. Limited availability of clinical trials at community oncology practices is a major contributor to outcome disparities among minorities, rural, and elderly patients, all of whom are underrepresented in clinical trials. Between 2003 and 2023, the National Cancer Institute (NCI) established programs to address these challenges: the Community Clinical Oncology Program, Minority- Based Community Clinical Oncology Program, NCI Community Cancer Centers Program, and NCI Community Oncology Research Program. However, disparities have persisted, particularly for pharmaceutical-directed clinical research. Lack of representation in clinical research results in data absenteeism, data chauvinism and hallucination, and a delay in treatment availability for high-risk hematologic malignancies in community practice. To address this, the US Congress enacted the Food and Drug Administration Omnibus Act in 2022 to help establish diversity plans that would broaden clinical trial patient enrollment in the United States. We recommend using these initiatives in community oncology practices, including the adoption of the DRIVE strategy in collaboration with pharmaceutical companies, as well as using the NCI-established programs to promote clinical trial availability for patients with high-risk malignancies treated in community oncology practices.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Maya N. Birhiray is the daughter of Ruemu E. Birhiray.
Ruemu Ejedafeta Birhiray: no competing financial interests to declare.
Maya Nicole Birhiray: no competing financial interests to declare.
Figures

References
-
- American Cancer Society Cancer Statistics Center. Estimated new cases 2023. https://cancerstatisticscenter.cancer.org/#!/data-analysis/NewCaseEstimated. Accessed 29 May 2023.
-
- Leukemia and Lymphoma Society. Facts and statistics overview, general blood cancers. https://www.lls.org/facts-and-statistics/facts-and-statistics-overview. Accessed 27 May 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

