Assessing Cannabidiol as a Therapeutic Agent for Preventing and Alleviating Alzheimer's Disease Neurodegeneration
- PMID: 38067101
- PMCID: PMC10705747
- DOI: 10.3390/cells12232672
Assessing Cannabidiol as a Therapeutic Agent for Preventing and Alleviating Alzheimer's Disease Neurodegeneration
Abstract
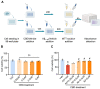
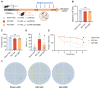
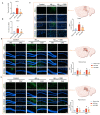
Alzheimer's disease (AD) is a leading neurodegenerative condition causing cognitive and memory decline. With small-molecule drugs targeting Aβ proving ineffective, alternative targets are urgently needed. Neuroinflammation, which is central to AD's pathology, results in synaptic and neuronal damage, highlighting the importance of addressing inflammation and conserving neuronal integrity. Cannabidiol (CBD), derived from cannabis, is noted for its neuroprotective and anti-inflammatory properties, having shown efficacy in neuropathic pain management for epilepsy. To investigate the therapeutic efficacy of CBD in AD and to elucidate its underlying mechanisms, we aimed to contribute valuable insights for incorporating AD prevention recommendations into future CBD nutritional guidelines. Aβ1-42 was employed for in vivo or in vitro model establishment, CBD treatment was utilized to assess the therapeutic efficacy of CBD, and RNA-seq analysis was conducted to elucidate the underlying therapeutic mechanism. CBD mitigates Aβ-induced cognitive deficits by modulating microglial activity, promoting neurotrophic factor release, and regulating inflammatory genes. The administration of CBD demonstrated a protective effect against Aβ toxicity both in vitro and in vivo, along with an amelioration of cognitive impairment in mice. These findings support the potential inclusion of CBD in future nutritional guidelines for Alzheimer's disease prevention.
Keywords: Alzheimer’s disease; anti-inflammatory; astrocyte; cannabidiol; microglia; neuroprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Cannabidiol as a multifaceted therapeutic agent: mitigating Alzheimer's disease pathology and enhancing cognitive function.Alzheimers Res Ther. 2025 May 20;17(1):109. doi: 10.1186/s13195-025-01756-0. Alzheimers Res Ther. 2025. PMID: 40394655 Free PMC article.
-
Chronic Treatment with 50 mg/kg Cannabidiol Improves Cognition and Moderately Reduces Aβ40 Levels in 12-Month-Old Male AβPPswe/PS1ΔE9 Transgenic Mice.J Alzheimers Dis. 2020;74(3):937-950. doi: 10.3233/JAD-191242. J Alzheimers Dis. 2020. PMID: 32116258
-
Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer's disease.Eur J Med Chem. 2020 Apr 15;192:112163. doi: 10.1016/j.ejmech.2020.112163. Epub 2020 Feb 22. Eur J Med Chem. 2020. PMID: 32109623 Review.
-
Cannabidiol improves the cognitive function of SAMP8 AD model mice involving the microbiota-gut-brain axis.J Toxicol Environ Health A. 2024 Jun 2;87(11):471-479. doi: 10.1080/15287394.2024.2338914. Epub 2024 Apr 9. J Toxicol Environ Health A. 2024. PMID: 38590254
-
Alzheimer's disease, aging, and cannabidiol treatment: a promising path to promote brain health and delay aging.Mol Biol Rep. 2024 Jan 16;51(1):121. doi: 10.1007/s11033-023-09162-1. Mol Biol Rep. 2024. PMID: 38227160 Review.
Cited by
-
Cannabidiol, a plant-derived compound, is an emerging strategy for treating cognitive impairments: comprehensive review of randomized trials.Front Pharmacol. 2024 Sep 11;15:1403147. doi: 10.3389/fphar.2024.1403147. eCollection 2024. Front Pharmacol. 2024. PMID: 39323633 Free PMC article. Review.
-
A systematic study of molecular targets of cannabidiol in Alzheimer's disease.J Alzheimers Dis Rep. 2024 Oct 11;8(1):1339-1360. doi: 10.1177/25424823241284464. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 40034365 Free PMC article. Review.
-
Opioid and Cannabinoid Systems in Pain: Emerging Molecular Mechanisms and Use in Clinical Practice, Health, and Fitness.Int J Mol Sci. 2024 Aug 29;25(17):9407. doi: 10.3390/ijms25179407. Int J Mol Sci. 2024. PMID: 39273354 Free PMC article. Review.
-
Future Therapeutic Strategies for Alzheimer's Disease: Focus on Behavioral and Psychological Symptoms.Int J Mol Sci. 2024 Oct 22;25(21):11338. doi: 10.3390/ijms252111338. Int J Mol Sci. 2024. PMID: 39518892 Free PMC article. Review.
-
Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer's Disease.Curr Issues Mol Biol. 2024 May 6;46(5):4379-4402. doi: 10.3390/cimb46050266. Curr Issues Mol Biol. 2024. PMID: 38785534 Free PMC article. Review.
References
-
- Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H., Cedazo-Minguez A., Dubois B., Edvardsson D., Feldman H., et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

