Non-Invasive Evaluation of Retinal Vascular Alterations in a Mouse Model of Optic Neuritis Using Laser Speckle Flowgraphy and Optical Coherence Tomography Angiography
- PMID: 38067113
- PMCID: PMC10705764
- DOI: 10.3390/cells12232685
Non-Invasive Evaluation of Retinal Vascular Alterations in a Mouse Model of Optic Neuritis Using Laser Speckle Flowgraphy and Optical Coherence Tomography Angiography
Abstract
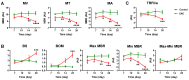
Optic neuritis, a characteristic feature of multiple sclerosis (MS), involves the inflammation of the optic nerve and the degeneration of retinal ganglion cells (RGCs). Although previous studies suggest that retinal blood flow alterations occur during optic neuritis, the precise location, the degree of impairment, and the underlying mechanisms remain unclear. In this study, we utilized two emerging non-invasive imaging techniques, laser speckle flowgraphy (LSFG) and optical coherence tomography angiography (OCTA), to investigate retinal vascular changes in a mouse model of MS, known as experimental autoimmune encephalomyelitis (EAE). We associated these changes with leukostasis, RGC injury, and the overall progression of EAE. LSFG imaging revealed a progressive reduction in retinal blood flow velocity and increased vascular resistance near the optic nerve head in the EAE model, indicating impaired ocular blood flow. OCTA imaging demonstrated significant decreases in vessel density, number of junctions, and total vessel length in the intermediate and deep capillary plexus of the EAE mice. Furthermore, our analysis of leukostasis revealed a significant increase in adherent leukocytes in the retinal vasculature of the EAE mice, suggesting the occurrence of vascular inflammation in the early development of EAE pathology. The abovechanges preceded or were accompanied by the characteristic hallmarks of optic neuritis, such as RGC loss and reduced visual acuity. Overall, our study sheds light on the intricate relationship between retinal vascular alterations and the progression of optic neuritis as well as MS clinical score. It also highlights the potential for the development of image-based biomarkers for the diagnosis and monitoring of optic neuritis as well as MS, particularly in response to emerging treatments.
Keywords: biomarker; laser speckle flowgraphy (LSFG); multiple sclerosis (MS); optic neuritis; optical coherence tomography angiography (OCTA); retinal blood flow; retinal vascular inflammation; retinal vasculature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Exploring experimental autoimmune optic neuritis using multimodal imaging.Neuroimage. 2018 Jul 15;175:327-339. doi: 10.1016/j.neuroimage.2018.04.004. Epub 2018 Apr 6. Neuroimage. 2018. PMID: 29627590
-
Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis.Mol Vis. 2016 Dec 30;22:1503-1513. eCollection 2016. Mol Vis. 2016. PMID: 28050123 Free PMC article.
-
Retinal pathology in experimental optic neuritis is characterized by retrograde degeneration and gliosis.Acta Neuropathol Commun. 2019 Jul 17;7(1):116. doi: 10.1186/s40478-019-0768-5. Acta Neuropathol Commun. 2019. PMID: 31315675 Free PMC article.
-
[Nonarteritic ischemic optic neuropathy animal model and its treatment applications].Nippon Ganka Gakkai Zasshi. 2014 Apr;118(4):331-61. Nippon Ganka Gakkai Zasshi. 2014. PMID: 24864434 Review. Japanese.
-
Assessing the anterior visual pathway in optic neuritis: recent experimental and clinical aspects.Curr Opin Neurol. 2019 Jun;32(3):346-357. doi: 10.1097/WCO.0000000000000675. Curr Opin Neurol. 2019. PMID: 30694926 Review.
Cited by
-
Evaluating Fundoscopy as a Screening Tool for Optic Nerve Atrophy in Multiple Sclerosis: An Optical Coherence Tomography (OCT) Comparative Study.J Clin Med. 2025 Mar 22;14(7):2166. doi: 10.3390/jcm14072166. J Clin Med. 2025. PMID: 40217617 Free PMC article.
-
Tissue Hypoxia and Associated Innate Immune Factors in Experimental Autoimmune Optic Neuritis.Int J Mol Sci. 2024 Mar 6;25(5):3077. doi: 10.3390/ijms25053077. Int J Mol Sci. 2024. PMID: 38474322 Free PMC article.
-
Noninvasive monitoring of vascular alterations in mice with acute lower limb ischemia using multimodal photoacoustic imaging.Bioeng Transl Med. 2025 Feb 17;10(4):e70005. doi: 10.1002/btm2.70005. eCollection 2025 Jul. Bioeng Transl Med. 2025. PMID: 40708973 Free PMC article.
References
-
- Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., Van Der Mei I., et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020;26:1816–1821. doi: 10.1177/1352458520970841. - DOI - PMC - PubMed
-
- De Heredia-Torres M.P., Huertas-Hoyas E., Sánchez-Camarero C., Máximo-Bocanegra N., Alegre-Ayala J., Sánchez-Herrera-Baeza P., Martínez-Piédrola R.M., García-Bravo C., Mayoral-Martín A., Serrada-Tejeda S. Occupational performance in multiple sclerosis and its relationship with quality of life and fatigue. Eur. J. Phys. Rehabil. Med. 2020;56:148–154. doi: 10.23736/S1973-9087.20.05914-6. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

