Calreticulin Regulates SARS-CoV-2 Spike Protein Turnover and Modulates SARS-CoV-2 Infectivity
- PMID: 38067122
- PMCID: PMC10705507
- DOI: 10.3390/cells12232694
Calreticulin Regulates SARS-CoV-2 Spike Protein Turnover and Modulates SARS-CoV-2 Infectivity
Abstract
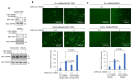
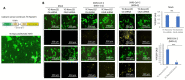
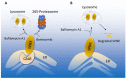
Cardiovascular complications are major clinical hallmarks of acute and post-acute coronavirus disease 2019 (COVID-19). However, the mechanistic details of SARS-CoV-2 infectivity of endothelial cells remain largely unknown. Here, we demonstrate that the receptor binding domain (RBD) of the SARS-CoV-2 spike (S) protein shares a similarity with the proline-rich binding ena/VASP homology (EVH1) domain and identified the endoplasmic reticulum (ER) resident calreticulin (CALR) as an S-RBD interacting protein. Our biochemical analysis showed that CALR, via its proline-rich (P) domain, interacts with S-RBD and modulates proteostasis of the S protein. Treatment of cells with the proteasomal inhibitor bortezomib increased the expression of the S protein independent of CALR, whereas the lysosomal/autophagy inhibitor bafilomycin 1A, which interferes with the acidification of lysosome, selectively augmented the S protein levels in a CALR-dependent manner. More importantly, the shRNA-mediated knockdown of CALR increased SARS-CoV-2 infection and impaired calcium homeostasis of human endothelial cells. This study provides new insight into the infectivity of SARS-CoV-2, calcium hemostasis, and the role of CALR in the ER-lysosome-dependent proteolysis of the spike protein, which could be associated with cardiovascular complications in COVID-19 patients.
Keywords: COVID-19; S-RBD; SARS-CoV-2; calreticulin; endothelial cells; intracellular calcium homeostasis; spike protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Amraei R., Xia C., Olejnik J., White M.R., Napoleon M.A., Lotfollahzadeh S., Hauser B.M., Schmidt A.G., Chitalia V., Muhlberger E., et al. Extracellular vimentin is an attachment factor that facilitates SARS-CoV-2 entry into human endothelial cells. Proc. Natl. Acad. Sci. USA. 2022;119:e2113874119. doi: 10.1073/pnas.2113874119. - DOI - PMC - PubMed
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

