Effects of Dickkopf-1 (DKK-1) on Prostate Cancer Growth and Bone Metastasis
- PMID: 38067123
- PMCID: PMC10705757
- DOI: 10.3390/cells12232695
Effects of Dickkopf-1 (DKK-1) on Prostate Cancer Growth and Bone Metastasis
Abstract
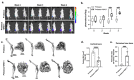
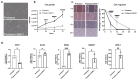
Osteoblastic bone metastases are commonly detected in patients with advanced prostate cancer (PCa) and are associated with an increased mortality rate. Dickkopf-1 (DKK-1) antagonizes canonical WNT/β-catenin signaling and plays a complex role in bone metastases. We explored the function of cancer cell-specific DKK-1 in PCa growth, metastasis, and cancer-bone interactions using the osteoblastic canine PCa cell line, Probasco. Probasco or Probasco + DKK-1 (cells transduced with human DKK-1) were injected into the tibia or left cardiac ventricle of athymic nude mice. Bone metastases were detected by bioluminescent imaging in vivo and evaluated by micro-computed tomography and histopathology. Cancer cell proliferation, migration, gene/protein expression, and their impact on primary murine osteoblasts and osteoclasts, were evaluated in vitro. DKK-1 increased cancer growth and stimulated cell migration independent of canonical WNT signaling. Enhanced cancer progression by DKK-1 was associated with increased cell proliferation, up-regulation of NF-kB/p65 signaling, inhibition of caspase-dependent apoptosis by down-regulation of non-canonical WNT/JNK signaling, and increased expression of epithelial-to-mesenchymal transition genes. In addition, DKK-1 attenuated the osteoblastic activity of Probasco cells, and bone metastases had decreased cancer-induced intramedullary woven bone formation. Decreased bone formation might be due to the inhibition of osteoblast differentiation and stimulation of osteoclast activity through a decrease in the OPG/RANKL ratio in the bone microenvironment. The present study indicated that the cancer-promoting role of DKK-1 in PCa bone metastases was associated with increased growth of bone metastases, reduced bone induction, and altered signaling through the canonical WNT-independent pathway. DKK-1 could be a promising therapeutic target for PCa.
Keywords: DKK-1; canine; dog; osteoblastic bone metastasis; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases.Prostate. 2011 May;71(6):615-25. doi: 10.1002/pros.21277. Epub 2010 Oct 18. Prostate. 2011. PMID: 20957670 Free PMC article.
-
Canine prostatic cancer cell line (LuMa) with osteoblastic bone metastasis.Prostate. 2020 Jun;80(9):698-714. doi: 10.1002/pros.23983. Epub 2020 Apr 29. Prostate. 2020. PMID: 32348616 Free PMC article.
-
p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1.Cancer Res. 2010 Dec 1;70(23):9916-26. doi: 10.1158/0008-5472.CAN-10-0440. Epub 2010 Nov 23. Cancer Res. 2010. PMID: 21098705 Free PMC article.
-
Role of Wnts in prostate cancer bone metastases.J Cell Biochem. 2006 Mar 1;97(4):661-72. doi: 10.1002/jcb.20735. J Cell Biochem. 2006. PMID: 16447163 Review.
-
Dickkopf-1: a suitable target for the management of myeloma bone disease.Expert Opin Ther Targets. 2009 Jul;13(7):839-48. doi: 10.1517/14728220903025770. Expert Opin Ther Targets. 2009. PMID: 19530987 Review.
Cited by
-
Bone metastases of prostate cancer: Molecular mechanisms, targeted diagnosis and targeted therapy (Review).Oncol Rep. 2025 Apr;53(4):46. doi: 10.3892/or.2025.8879. Epub 2025 Feb 21. Oncol Rep. 2025. PMID: 39981932 Free PMC article. Review.
-
Ferroptosis enhances the therapeutic potential of oncolytic adenoviruses KD01 against cancer.Cancer Gene Ther. 2025 Apr;32(4):403-417. doi: 10.1038/s41417-025-00882-z. Epub 2025 Mar 3. Cancer Gene Ther. 2025. PMID: 40033102 Free PMC article.
-
Tetramethylpyrazine alleviates acute kidney injury by activating the Wnt/β-catenin pathway independent of DKK1.Exp Ther Med. 2025 Aug 27;30(5):208. doi: 10.3892/etm.2025.12958. eCollection 2025 Nov. Exp Ther Med. 2025. PMID: 40927640 Free PMC article.
-
Bridging the Gap in Understanding Bone Metastasis: A Multifaceted Perspective.Int J Mol Sci. 2024 Feb 29;25(5):2846. doi: 10.3390/ijms25052846. Int J Mol Sci. 2024. PMID: 38474093 Free PMC article. Review.
-
Targeting cancer-induced skeletal damage: a holistic approach to understanding pathophysiology, mechanisms, and management solutions.Am J Cancer Res. 2025 Apr 15;15(4):1494-1516. doi: 10.62347/QFHJ2430. eCollection 2025. Am J Cancer Res. 2025. PMID: 40371144 Free PMC article. Review.
References
-
- Dai J., Kitagawa Y., Zhang J., Yao Z., Mizokami A., Cheng S., Nor J., McCauley L.K., Taichman R.S., Keller E.T. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64:994–999. doi: 10.1158/0008-5472.CAN-03-1382. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

