Cytological Study of Topical Effect of Azelastine Hydrochloride on the Nasal Mucous Membrane Cells in Various Nasal Rhinitis Types
- PMID: 38067125
- PMCID: PMC10706206
- DOI: 10.3390/cells12232697
Cytological Study of Topical Effect of Azelastine Hydrochloride on the Nasal Mucous Membrane Cells in Various Nasal Rhinitis Types
Abstract
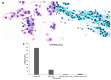
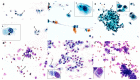
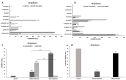
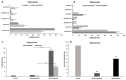
Previous reports on the benefits of using local therapy with azelastine in rhinitis focus on the assessment of clinical symptoms and the analysis of nasal lavage for the presence of inflammatory cells and the expression of adhesion molecules. Little attention has been paid to studies assessing the effect of azelastine on individual cytotypes of the nasal mucosa, especially epithelial cells, also in the context of inducing morphological changes. The aim of this study was the cytological analysis of swabs taken from the surface of the nasal mucosa of patients with allergic rhinitis (AR) and nonallergic/vasomotor rhinitis (NAR/VMR) who were subjected to 4 weeks of therapy with azelastine and then comparing the obtained results with the pre-treatment condition. The technique of obtaining materials for cytoanalysis included sampling, staining of smears, microscopic analysis, and preparation of cytograms. Our studies confirmed the therapeutic benefits of azelastine in both study groups. Significant changes were demonstrated, confirming the regeneration of ciliated cells and the induction of autophagy and apoptosis in epithelial cells. Such changes indicate new mechanisms of action of azelastine, which play a significant role in restoring homeostasis in the nasal mucosa. The presented research also results in a detailed description of cytological changes in both studied rhinitis types, which complements the knowledge regarding prognostic indicators.
Keywords: azelastine hydrochloride; nasal cytology; rhinitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Effect of intranasal azelastine on substance P release in perennial nonallergic rhinitis patients.Am J Rhinol Allergy. 2013 Nov-Dec;27(6):514-6. doi: 10.2500/ajra.2013.27.3955. Am J Rhinol Allergy. 2013. PMID: 24274229
-
Open-label evaluation of azelastine nasal spray in patients with seasonal allergic rhinitis and nonallergic vasomotor rhinitis.Curr Med Res Opin. 2005 Apr;21(4):611-8. doi: 10.1185/030079905X41408. Curr Med Res Opin. 2005. PMID: 15899111 Clinical Trial.
-
Azelastine hydrochloride: a review of pharmacology, pharmacokinetics, clinical efficacy and tolerability.Curr Med Res Opin. 2007 Oct;23(10):2441-52. doi: 10.1185/030079907X226302. Curr Med Res Opin. 2007. PMID: 17723160 Review.
-
Azelastine nasal spray: a review of pharmacology and clinical efficacy in allergic and nonallergic rhinitis.Allergy Asthma Proc. 2003 Mar-Apr;24(2):95-105. Allergy Asthma Proc. 2003. PMID: 12776442 Review.
-
Azelastine nasal spray inhibiting sympathetic function on human nasal mucosa in patients with allergy rhinitis.Rhinology. 2019 Aug 1;57(4):268-272. doi: 10.4193/Rhin18.274. Rhinology. 2019. PMID: 30887967
Cited by
-
Administration Strategy-Dependent Mechanisms and Effects of Human Adipose Tissue Stem Cell Extracellular Vesicles in Mouse Allergic Rhinitis Treatment.Cell Transplant. 2025 Jan-Dec;34:9636897251325673. doi: 10.1177/09636897251325673. Epub 2025 Apr 3. Cell Transplant. 2025. PMID: 40179013 Free PMC article.
References
-
- Procopiou P.A., Ford A.J., Gore P.M., Looker B.E., Hodgson S.T., Holmes D.S., Vile S., Clark K.L., Saunders K.A., Slack R.J., et al. Design of Phthalazinone Amide Histamine H(1) Receptor Antagonists for Use in Rhinitis. ACS Med. Chem. Lett. 2017;8:577–581. doi: 10.1021/acsmedchemlett.7b00112. - DOI - PMC - PubMed
-
- Ellis A.K., Zhu Y., Steacy L.M., Walker T., Day J.H. A four-way, double-blind, randomized, placebo controlled study to determine the efficacy and speed of azelastine nasal spray, versus loratadine, and cetirizine in adult subjects with allergen-induced seasonal allergic rhinitis. Allergy Asthma Clin. Immunol. 2013;9:16. doi: 10.1186/1710-1492-9-16. - DOI - PMC - PubMed
-
- Horbal J.M., Bernstein J.A. Azelastine HCl: A Review of the Old and New Formulations. Clin. Med. Insights Ther. 2010;2:427–437. doi: 10.4137/CMT.S3865. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

