An Exosome-Rich Conditioned Medium from Human Amniotic Membrane Stem Cells Facilitates Wound Healing via Increased Reepithelization, Collagen Synthesis, and Angiogenesis
- PMID: 38067126
- PMCID: PMC10705799
- DOI: 10.3390/cells12232698
An Exosome-Rich Conditioned Medium from Human Amniotic Membrane Stem Cells Facilitates Wound Healing via Increased Reepithelization, Collagen Synthesis, and Angiogenesis
Abstract
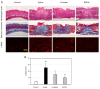
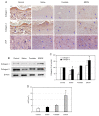
Tissue regeneration is an essential requirement for wound healing and recovery of organs' function. It has been demonstrated that wound healing can be facilitated by activating paracrine signaling mediated by exosomes secreted from stem cells, since exosomes deliver many functional molecules including growth factors (GFs) and neurotrophic factors (NFs) effective for tissue regeneration. In this study, an exosome-rich conditioned medium (ERCM) was collected from human amniotic membrane stem cells (AMSCs) by cultivating the cells under a low oxygen tension (2% O2 and 5% CO2). The contents of GFs and NFs including keratinocyte growth factor, epidermal growth factor, fibroblast growth factor 1, transforming growth factor-β, and vascular endothelial growth factor responsible for skin regeneration were much higher (10-30 folds) in the ERCM than in normal conditioned medium (NCM). In was found that CM-DiI-labeled exosomes readily entered keratinocytes and fibroblasts, and that ERCM not only facilitated the proliferation of keratinocytes in normal condition, but also protected against H2O2 cytotoxicity. In cell-migration assay, the scratch wound in keratinocyte culture dish was rapidly closed by treatment with ERCM. Such wound-healing effects of ERCM were confirmed in a rat whole skin-excision model: i.e., the wound closure was significantly accelerated, remaining minimal crusts, by topical application of ERCM solution (4 × 109 exosome particles/100 μL) at 4-day intervals. In the wounded skin, the deposition of collagens was enhanced by treatment with ERCM, which was supported by the increased production of collagen-1 and collagen-3. In addition, enhanced angiogenesis in ERCM-treated wounds was confirmed by increased von Willebrand factor (vWF)-positive endothelial cells. The results indicate that ERCM from AMSCs with high concentrations of GFs and NFs improves wound healing through tissue regeneration not only by facilitating keratinocyte proliferation for skin repair, but also activating fibroblasts for extracellular matrix production, in addition to the regulation of angiogenesis and scar tissue formation.
Keywords: amniotic membrane stem cell; angiogenesis; collagen synthesis; exosome-rich conditioned medium (ERCM); growth factor; keratinocyte proliferation; neurotrophic factor; wound healing.
Conflict of interest statement
Authors Chan Ho Noh, Sangryong Park, Hye-Rim Seong, Ah-young Lee, Tae Myoung Kim, Ehn-Kyoung Choi and Yun-Bae Kim were employed by the company Designed Cells Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Intraocular Pressure-Lowering and Retina-Protective Effects of Exosome-Rich Conditioned Media from Human Amniotic Membrane Stem Cells in a Rat Model of Glaucoma.Int J Mol Sci. 2023 Apr 29;24(9):8073. doi: 10.3390/ijms24098073. Int J Mol Sci. 2023. PMID: 37175778 Free PMC article.
-
Extracellular matrix/stromal vascular fraction gel conditioned medium accelerates wound healing in a murine model.Wound Repair Regen. 2017 Nov;25(6):923-932. doi: 10.1111/wrr.12602. Epub 2018 Feb 6. Wound Repair Regen. 2017. PMID: 29240284
-
Preventive Effects of Exosome-Rich Conditioned Medium From Amniotic Membrane-Derived Mesenchymal Stem Cells for Diabetic Retinopathy in Rats.Transl Vis Sci Technol. 2023 Aug 1;12(8):18. doi: 10.1167/tvst.12.8.18. Transl Vis Sci Technol. 2023. PMID: 37610767 Free PMC article.
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
-
The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review.Stem Cell Res Ther. 2022 Jan 24;13(1):24. doi: 10.1186/s13287-021-02697-9. Stem Cell Res Ther. 2022. PMID: 35073970 Free PMC article. Review.
Cited by
-
Effects of Chlorella protothecoides-derived polydeoxyribonucleotides on skin regeneration and wound healing.Arch Dermatol Res. 2025 Feb 24;317(1):483. doi: 10.1007/s00403-025-03885-w. Arch Dermatol Res. 2025. PMID: 39994014
-
Microenvironmental Modulation for Therapeutic Efficacy of Extracellular Vesicles.Adv Sci (Weinh). 2025 May;12(18):e2503027. doi: 10.1002/advs.202503027. Epub 2025 Mar 27. Adv Sci (Weinh). 2025. PMID: 40145773 Free PMC article. Review.
-
Exploring the dermatological applications of human mesenchymal stem cell secretome: a comprehensive review.Stem Cell Res Ther. 2025 Apr 12;16(1):177. doi: 10.1186/s13287-025-04311-8. Stem Cell Res Ther. 2025. PMID: 40221781 Free PMC article. Review.
-
Milk-derived exosomes as functional nanocarriers in wound healing: Mechanisms, applications, and future directions.Mater Today Bio. 2025 Mar 28;32:101715. doi: 10.1016/j.mtbio.2025.101715. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40242483 Free PMC article. Review.
-
Exosome Source Matters: A Comprehensive Review from the Perspective of Diverse Cellular Origins.Pharmaceutics. 2025 Jan 22;17(2):147. doi: 10.3390/pharmaceutics17020147. Pharmaceutics. 2025. PMID: 40006514 Free PMC article. Review.
References
-
- Van den Steen P.E., Proost P., Wuyts A., Van Damme J., Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by amino terminal processing, where a sit degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. doi: 10.1182/blood.V96.8.2673. - DOI - PubMed
-
- Karsdal M.A., Larsen L., Engsig M.T., Lou H., Ferreras M., Lochter A., Delaissé J.M., Foged N.T. Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 2002;277:44061–44067. doi: 10.1074/jbc.M207205200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

