Antifibrotic Effect of Selenium-Containing Nanoparticles on a Model of TAA-Induced Liver Fibrosis
- PMID: 38067151
- PMCID: PMC10706216
- DOI: 10.3390/cells12232723
Antifibrotic Effect of Selenium-Containing Nanoparticles on a Model of TAA-Induced Liver Fibrosis
Abstract
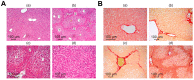
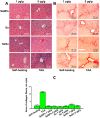
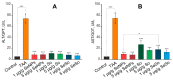
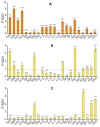
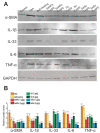
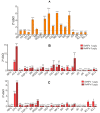
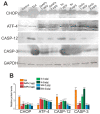
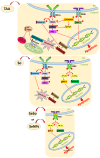
For the first time, based on the expression analysis of a wide range of pro- and anti-fibrotic, pro- and anti-inflammatory, and pro- and anti-apoptotic genes, key markers of endoplasmic reticulum stress (ER-stress), molecular mechanisms for the regulation of fibrosis, and accompanying negative processes caused by thioacetamide (TAA) injections and subsequent injections of selenium-containing nanoparticles and sorafenib have been proposed. We found that selenium nanoparticles of two types (doped with and without sorafenib) led to a significant decrease in almost all pro-fibrotic and pro-inflammatory genes. Sorafenib injections also reduced mRNA expression of pro-fibrotic and pro-inflammatory genes but less effectively than both types of nanoparticles. In addition, it was shown for the first time that TAA can be an inducer of ER-stress, most likely activating the IRE1α and PERK signaling pathways of the UPR, an inducer of apoptosis and pyroptosis. Sorafenib, despite a pronounced anti-apoptotic effect, still did not reduce the expression of caspase-3 and 12 or mitogen-activated kinase JNK1 to control values, which increases the risk of persistent apoptosis in liver cells. After injections of selenium-containing nanoparticles, the negative effects caused by TAA were leveled, causing an adaptive UPR signaling response through activation of the PERK signaling pathway. The advantages of selenium-containing nanoparticles over sorafenib, established in this work, once again emphasize the unique properties of this microelement and serve as an important factor for the further introduction of drugs based on it into clinical practice.
Keywords: apoptosis; liver fibrosis; selenium; selenium nanoparticles; sorafenib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
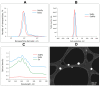
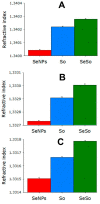
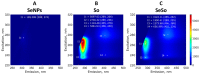

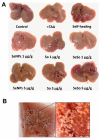





Similar articles
-
Celecoxib attenuates hepatocyte apoptosis by inhibiting endoplasmic reticulum stress in thioacetamide-induced cirrhotic rats.World J Gastroenterol. 2020 Jul 28;26(28):4094-4107. doi: 10.3748/wjg.v26.i28.4094. World J Gastroenterol. 2020. PMID: 32821072 Free PMC article.
-
Selenium nanoparticles overcomes sorafenib resistance in thioacetamide induced hepatocellular carcinoma in rats by modulation of mTOR, NF-κB pathways and LncRNA-AF085935/GPC3 axis.Life Sci. 2022 Aug 15;303:120675. doi: 10.1016/j.lfs.2022.120675. Epub 2022 May 28. Life Sci. 2022. PMID: 35640776
-
Comparative Analysis of the Cytotoxic Effect of a Complex of Selenium Nanoparticles Doped with Sorafenib, "Naked" Selenium Nanoparticles, and Sorafenib on Human Hepatocyte Carcinoma HepG2 Cells.Int J Mol Sci. 2022 Jun 14;23(12):6641. doi: 10.3390/ijms23126641. Int J Mol Sci. 2022. PMID: 35743086 Free PMC article.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
Molecular Basis of Human Diseases and Targeted Therapy Based on Small-Molecule Inhibitors of ER Stress-Induced Signaling Pathways.Curr Mol Med. 2017;17(2):118-132. doi: 10.2174/1566524017666170306122643. Curr Mol Med. 2017. PMID: 28266275 Review.
Cited by
-
Exploring the Mechanisms of Iron Overload-Induced Liver Injury in Rats Based on Transcriptomics and Proteomics.Biology (Basel). 2025 Jan 16;14(1):81. doi: 10.3390/biology14010081. Biology (Basel). 2025. PMID: 39857310 Free PMC article.
-
Advances in Selenium and Related Compounds Inhibiting Multi-Organ Fibrosis.Drug Des Devel Ther. 2025 Jan 14;19:251-265. doi: 10.2147/DDDT.S488226. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 39830783 Free PMC article. Review.
-
Liver cirrhosis: molecular mechanisms and therapeutic interventions.MedComm (2020). 2024 Sep 17;5(10):e721. doi: 10.1002/mco2.721. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39290252 Free PMC article. Review.
-
Opposite Effects of Small and Large Diameter Selenium Nanoparticles on the Redox-Status and Survival of Cortical Cells in Toxic Models In Vitro.Biol Trace Elem Res. 2025 May 29. doi: 10.1007/s12011-025-04682-2. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40442457
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

