Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans
- PMID: 38067156
- PMCID: PMC10705807
- DOI: 10.3390/cells12232728
Life-Cycle-Dependent Toxicities of Mono- and Bifunctional Alkylating Agents in the 3R-Compliant Model Organism C. elegans
Abstract
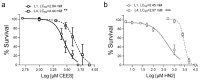
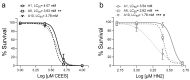
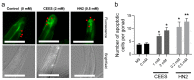
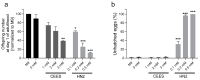
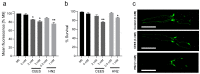
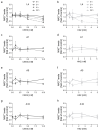
Caenorhabditis elegans (C. elegans) is gaining recognition and importance as an organismic model for toxicity testing in line with the 3Rs principle (replace, reduce, refine). In this study, we explored the use of C. elegans to examine the toxicities of alkylating sulphur mustard analogues, specifically the monofunctional agent 2-chloroethyl-ethyl sulphide (CEES) and the bifunctional, crosslinking agent mechlorethamine (HN2). We exposed wild-type worms at different life cycle stages (from larvae L1 to adulthood day 10) to CEES or HN2 and scored their viability 24 h later. The susceptibility of C. elegans to CEES and HN2 paralleled that of human cells, with HN2 exhibiting higher toxicity than CEES, reflected in LC50 values in the high µM to low mM range. Importantly, the effects were dependent on the worms' developmental stage as well as organismic age: the highest susceptibility was observed in L1, whereas the lowest was observed in L4 worms. In adult worms, susceptibility to alkylating agents increased with advanced age, especially to HN2. To examine reproductive effects, L4 worms were exposed to CEES and HN2, and both the offspring and the percentage of unhatched eggs were assessed. Moreover, germline apoptosis was assessed by using ced-1p::GFP (MD701) worms. In contrast to concentrations that elicited low toxicities to L4 worms, CEES and HN2 were highly toxic to germline cells, manifesting as increased germline apoptosis as well as reduced offspring number and percentage of eggs hatched. Again, HN2 exhibited stronger effects than CEES. Compound specificity was also evident in toxicities to dopaminergic neurons-HN2 exposure affected expression of dopamine transporter DAT-1 (strain BY200) at lower concentrations than CEES, suggesting a higher neurotoxic effect. Mechanistically, nicotinamide adenine dinucleotide (NAD+) has been linked to mustard agent toxicities. Therefore, the NAD+-dependent system was investigated in the response to CEES and HN2 treatment. Overall NAD+ levels in worm extracts were revealed to be largely resistant to mustard exposure except for high concentrations, which lowered the NAD+ levels in L4 worms 24 h post-treatment. Interestingly, however, mutant worms lacking components of NAD+-dependent pathways involved in genome maintenance, namely pme-2, parg-2, and sirt-2.1 showed a higher and compound-specific susceptibility, indicating an active role of NAD+ in genotoxic stress response. In conclusion, the present results demonstrate that C. elegans represents an attractive model to study the toxicology of alkylating agents, which supports its use in mechanistic as well as intervention studies with major strength in the possibility to analyze toxicities at different life cycle stages.
Keywords: C. elegans; NAD+; alkylating agents; life cycle toxicities; mustards; neurotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
NAD+ Acts as a Protective Factor in Cellular Stress Response to DNA Alkylating Agents.Cells. 2023 Oct 2;12(19):2396. doi: 10.3390/cells12192396. Cells. 2023. PMID: 37830610 Free PMC article.
-
Comparative toxicity of mono- and bifunctional alkylating homologues of sulphur mustard in human skin keratinocytes.Toxicology. 2017 May 1;382:36-46. doi: 10.1016/j.tox.2017.03.005. Epub 2017 Mar 8. Toxicology. 2017. PMID: 28285101
-
Sulfur and nitrogen mustards induce characteristic poly(ADP-ribosyl)ation responses in HaCaT keratinocytes with distinctive cellular consequences.Toxicol Lett. 2016 Feb 26;244:56-71. doi: 10.1016/j.toxlet.2015.09.010. Epub 2015 Sep 14. Toxicol Lett. 2016. PMID: 26383629
-
Caenorhabditis elegans Model for Initial Screening and Mechanistic Evaluation of Potential New Drugs for Aging and Alzheimer’s Disease.In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 16. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 16. PMID: 21204333 Free Books & Documents. Review.
-
Caenorhabditis elegans as a Model to Study Manganese-Induced Neurotoxicity.Biomolecules. 2022 Sep 29;12(10):1396. doi: 10.3390/biom12101396. Biomolecules. 2022. PMID: 36291605 Free PMC article. Review.
Cited by
-
The Invertebrate Immunocyte: A Complex and Versatile Model for Immunological, Developmental, and Environmental Research.Cells. 2024 Dec 19;13(24):2106. doi: 10.3390/cells13242106. Cells. 2024. PMID: 39768196 Free PMC article. Review.
References
-
- Mack H.I.D., Heimbucher T., Murphy C.T. The nematode Caenorhabditis elegans as a model for aging research. Drug Discov. Today Dis. Models. 2018;27:3–13. doi: 10.1016/j.ddmod.2018.11.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

