Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis
- PMID: 38067157
- PMCID: PMC10706396
- DOI: 10.3390/cells12232730
Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis
Abstract
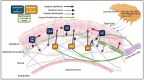
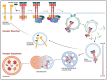
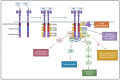
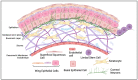
The corneal epithelium is the first anatomical barrier between the environment and the cornea; it is critical for proper light refraction onto the retina and prevents pathogens (e.g., bacteria, viruses) from entering the immune-privileged eye. Trauma to the highly innervated corneal epithelium is extremely painful and if not resolved quickly or properly, can lead to infection and ultimately blindness. The healthy eye produces its own growth factors and is continuously bathed in tear fluid that contains these proteins and other nutrients to maintain the rapid turnover and homeostasis of the ocular surface. In this article, we review the roles of growth factors in corneal epithelial homeostasis and regeneration and some of the limitations to their use therapeutically.
Keywords: c-Met; cornea; corneal epithelium; corneal nerves; growth factor; growth factor receptor; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epidermal Growth Factor Receptor Expression in the Corneal Epithelium.Cells. 2021 Sep 13;10(9):2409. doi: 10.3390/cells10092409. Cells. 2021. PMID: 34572058 Free PMC article. Review.
-
[The cornea: stasis and dynamics].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):179-212; discussion 213. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411711 Review. Japanese.
-
Corneal Epithelial Wound Healing.Prog Mol Biol Transl Sci. 2015;134:61-71. doi: 10.1016/bs.pmbts.2015.05.002. Epub 2015 Jun 12. Prog Mol Biol Transl Sci. 2015. PMID: 26310149 Review.
-
Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding.Exp Eye Res. 1999 Apr;68(4):377-97. doi: 10.1006/exer.1998.0603. Exp Eye Res. 1999. PMID: 10192796
-
Nerve growth factor and corneal wound healing in dogs.Exp Eye Res. 2005 May;80(5):633-42. doi: 10.1016/j.exer.2004.11.013. Epub 2005 Jan 4. Exp Eye Res. 2005. PMID: 15862170
Cited by
-
Comparison of Corneal Epitheliotrophic Factors of Undiluted Autologous Platelet-Rich Plasma and Autologous Serum Eye Drops for Dry Eye Disease.Ophthalmol Ther. 2025 Feb;14(2):363-377. doi: 10.1007/s40123-024-01082-y. Epub 2024 Dec 20. Ophthalmol Ther. 2025. PMID: 39704778 Free PMC article.
-
Regulation of epithelial growth factor receptors by the oncoprotein E5 during the HPV16 differentiation-dependent life cycle.Tumour Virus Res. 2025 Jun;19:200315. doi: 10.1016/j.tvr.2025.200315. Epub 2025 Mar 7. Tumour Virus Res. 2025. PMID: 40057277 Free PMC article. Review.
-
The Role of Insulin-like Growth Factor (IGF) System in the Corneal Epithelium Homeostasis-From Limbal Epithelial Stem Cells to Therapeutic Applications.Biology (Basel). 2024 Feb 25;13(3):144. doi: 10.3390/biology13030144. Biology (Basel). 2024. PMID: 38534414 Free PMC article. Review.
-
Neurotrophic keratopathy: Update in diagnosis and management.Indian J Ophthalmol. 2025 Apr 1;73(4):483-495. doi: 10.4103/IJO.IJO_2963_24. Epub 2025 Mar 27. Indian J Ophthalmol. 2025. PMID: 40146136 Free PMC article. Review.
-
LNP-encapsulated miRNA29b for corneal repair: A novel approach to combat fibrosis.Mater Today Bio. 2025 Mar 22;32:101695. doi: 10.1016/j.mtbio.2025.101695. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40230645 Free PMC article.
References
-
- Scott P. Scott’s Anatomy of the Eye and Orbit. Ridgevue Publishing; Atascadero, CA, USA: 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

