Human Placental Mesenchymal Stem Cells and Derived Extracellular Vesicles Ameliorate Lung Injury in Acute Respiratory Distress Syndrome Murine Model
- PMID: 38067158
- PMCID: PMC10706384
- DOI: 10.3390/cells12232729
Human Placental Mesenchymal Stem Cells and Derived Extracellular Vesicles Ameliorate Lung Injury in Acute Respiratory Distress Syndrome Murine Model
Abstract
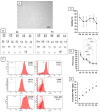
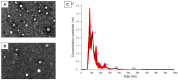
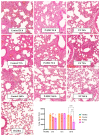
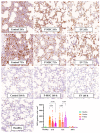
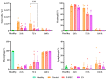
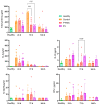
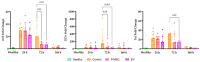
This study investigates the therapeutic potential of human placental mesenchymal stem cells (P-MSCs) and their extracellular vesicles (EVs) in a murine model of acute respiratory distress syndrome (ARDS), a condition with growing relevance due to its association with severe COVID-19. We induced ARDS-like lung injury in mice using intranasal LPS instillation and evaluated histological changes, neutrophil accumulation via immunohistochemistry, bronchoalveolar lavage fluid cell count, total protein, and cytokine concentration, as well as lung gene expression changes at three time points: 24, 72, and 168 h. We found that both P-MSCs and EV treatments reduced the histological evidence of lung injury, decreased neutrophil infiltration, and improved alveolar barrier integrity. Analyses of cytokines and gene expression revealed that both treatments accelerated inflammation resolution in lung tissue. Biodistribution studies indicated negligible cell engraftment, suggesting that intraperitoneal P-MSC therapy functions mostly through soluble factors. Overall, both P-MSC and EV therapy ameliorated LPS-induced lung injury. Notably, at the tested dose, EV therapy was more effective than P-MSCs in reducing most aspects of lung injury.
Keywords: COVID-19; acute respiratory distress syndrome (ARDS); exosomes; extracellular vesicles (EVs); good manufacturing practice (GMP); immunomodulation; inflammation; mesenchymal stem cells (MSCs); preclinical model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
MSC-NTF (NurOwn®) exosomes: a novel therapeutic modality in the mouse LPS-induced ARDS model.Stem Cell Res Ther. 2021 Jan 19;12(1):72. doi: 10.1186/s13287-021-02143-w. Stem Cell Res Ther. 2021. PMID: 33468250 Free PMC article.
-
GMP-compliant extracellular vesicles derived from umbilical cord mesenchymal stromal cells: manufacturing and pre-clinical evaluation in ARDS treatment.Cytotherapy. 2024 Sep;26(9):1013-1025. doi: 10.1016/j.jcyt.2024.04.074. Epub 2024 May 1. Cytotherapy. 2024. PMID: 38762805
-
Small Extracellular Vesicles Derived from Cord Blood Plasma and Placental Mesenchymal Stem Cells Attenuate Acute Lung Injury Induced by Lipopolysaccharide (LPS).Int J Mol Sci. 2024 Dec 25;26(1):75. doi: 10.3390/ijms26010075. Int J Mol Sci. 2024. PMID: 39795932 Free PMC article.
-
Effects of mesenchymal stromal cell-derived extracellular vesicles in acute respiratory distress syndrome (ARDS): Current understanding and future perspectives.J Leukoc Biol. 2021 Jul;110(1):27-38. doi: 10.1002/JLB.3MR0321-545RR. Epub 2021 May 6. J Leukoc Biol. 2021. PMID: 33955590 Free PMC article. Review.
-
Extracellular Vesicles Derived from Mesenchymal Stem Cells: A Potential Biodrug for Acute Respiratory Distress Syndrome Treatment.BioDrugs. 2022 Nov;36(6):701-715. doi: 10.1007/s40259-022-00555-5. Epub 2022 Sep 10. BioDrugs. 2022. PMID: 36087245 Free PMC article. Review.
Cited by
-
Long-term outcomes of mesenchymal stem cell therapy in severe COVID-19 patients: 3-year follow-up of a randomized, double-blind, placebo-controlled trial.Stem Cell Res Ther. 2025 Feb 25;16(1):94. doi: 10.1186/s13287-025-04148-1. Stem Cell Res Ther. 2025. PMID: 40001244 Free PMC article. Clinical Trial.
-
Safety, Efficacy and Bio-Distribution Analysis of Exosomes Derived From Human Umbilical Cord Mesenchymal Stem Cells for Effective Treatment of Bronchopulmonary Dysplasia by Intranasal Administration in Mice Model.Int J Nanomedicine. 2025 Feb 27;20:2521-2553. doi: 10.2147/IJN.S501843. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40034220 Free PMC article.
-
Mesenchymal stem cell treatment alleviates seawater drowning induced lung injury by inhibiting the TNFα/Snail/EMT pathway.J Thorac Dis. 2024 Nov 30;16(11):7836-7852. doi: 10.21037/jtd-24-1471. Epub 2024 Nov 25. J Thorac Dis. 2024. PMID: 39678856 Free PMC article.
-
Comparison of efficacy of exosomes derived from human umbilical cord blood mesenchymal stem cells in treating mouse acute lung injury via different routes.Front Pediatr. 2025 May 29;13:1560915. doi: 10.3389/fped.2025.1560915. eCollection 2025. Front Pediatr. 2025. PMID: 40510682 Free PMC article.
-
Progression of mesenchymal stem cell regulation on imbalanced microenvironment after spinal cord injury.Stem Cell Res Ther. 2024 Oct 1;15(1):343. doi: 10.1186/s13287-024-03914-x. Stem Cell Res Ther. 2024. PMID: 39354635 Free PMC article. Review.
References
-
- ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

