Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells
- PMID: 38067161
- PMCID: PMC10706043
- DOI: 10.3390/cells12232733
Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells
Abstract
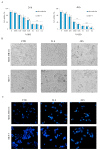
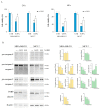
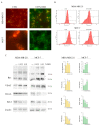
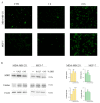
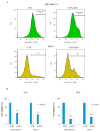
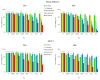
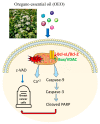
Origanum vulgare L. is an aromatic plant that exerts antibacterial, antioxidant, anti-inflammatory, and antitumor activities, mainly due to its essential oil (EO) content. In this study, we investigated the possible mechanism underlying the in vitro antitumor activity of EO extracted by hydrodistillation of dried flowers and leaves of Origanum vulgare L. grown in Sicily (Italy) in MDA-MB-231 and MCF-7 breast cancer cell lines. Gas chromatography-mass spectrometry analysis of Oregano essential oil (OEO) composition highlighted the presence of twenty-six major phytocompounds, such as p-cymene, γ-terpinene, and thymoquinone p-acetanisole. OEO possesses strong antioxidant capacity, as demonstrated by the DPPH test. Our studies provided evidence that OEO reduces the viability of both MCF-7 and MDA-MB-231 cells. The cytotoxic effect of OEO on breast cancer cells was partially counteracted by the addition of z-VAD-fmk, a general caspase inhibitor. Caspases and mitochondrial dysfunction appeared to be involved in the OEO-induced death mechanism. Western blotting analysis showed that OEO-induced activation of pro-caspases-9 and -3 and fragmentation of PARP decreased the levels of Bcl-2 and Bcl-xL while increasing those of Bax and VDAC. In addition, fluorescence microscopy and cytofluorimetric analysis showed that OEO induces a loss of mitochondrial membrane potential in both cell lines. Furthermore, we tested the effects of p-cymene, γ-terpinene, thymoquinone, and p-acetanisole, which are the main components of OEO. Our findings highlighted that the effect of OEO on MDA-MB-231 and MCF-7 cells appears to be mainly due to the combination of different constituents of OEO, providing evidence of the potential use of OEO for breast cancer treatment.
Keywords: GC–MS; antioxidant activity; breast cancer; essential oil; oregano.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Arabaci T., Çelenk S., Özcan T., Martin E., Yazici T., Açar M., Üzel D., Dirmenci T. Homoploid Hybrids of Origanum (Lamiaceae) in Turkey: Morphological and Molecular Evidence for a New Hybrid. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021;155:470–482. doi: 10.1080/11263504.2020.1762777. - DOI
-
- Harborne M.S., Jeffrey B. Oregano. CRC Press; Boca Raton, FL, USA: 2002. The Taxonomy and Chemistry of Origanum.
-
- Lotti C., Ricciardi L., Rainaldi G., Ruta C., Tarraf W., De Mastro G. Morphological, Biochemical, and Molecular Analysis of Origanum vulgare L. Open Agric. J. 2019;13:116–124. doi: 10.2174/1874331501913010116. - DOI
-
- Lombrea A., Antal D., Ardelean F., Avram S., Pavel I.Z., Vlaia L., Mut A.-M., Diaconeasa Z., Dehelean C.A., Soica C., et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020;21:9653. doi: 10.3390/ijms21249653. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

