Alteration of Immunoregulatory Patterns and Survival Advantage of Key Cell Types in Food Allergic Children
- PMID: 38067164
- PMCID: PMC10706629
- DOI: 10.3390/cells12232736
Alteration of Immunoregulatory Patterns and Survival Advantage of Key Cell Types in Food Allergic Children
Abstract
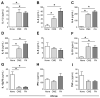
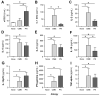
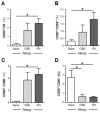
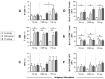
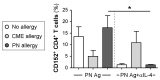
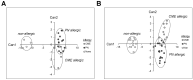
All allergic responses to food indicate the failure of immunological tolerance, but it is unclear why cow's milk and egg (CME) allergies resolve more readily than reactivity to peanuts (PN). We sought to identify differences between PN and CME allergies through constitutive immune status and responses to cognate and non-cognate food antigens. Children with confirmed allergy to CME (n = 6) and PN (n = 18) and non-allergic (NA) (n = 8) controls were studied. Constitutive secretion of cytokines was tested in plasma and unstimulated mononuclear cell (PBMNC) cultures. Blood dendritic cell (DC) subsets were analyzed alongside changes in phenotypes and soluble molecules in allergen-stimulated MNC cultures with or without cytokine neutralization. We observed that in allergic children, constitutively high plasma levels IL-1β, IL-2, IL-4, IL-5 and IL-10 but less IL-12p70 than in non-allergic children was accompanied by the spontaneous secretion of sCD23, IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IFN-γ and TNF-α in MNC cultures. Furthermore, blood DC subset counts differed in food allergy. Antigen-presenting cell phenotypic abnormalities were accompanied by higher B and T cell percentages with more Bcl-2 within CD69+ subsets. Cells were generally refractory to antigenic stimulation in vitro, but IL-4 neutralization led to CD152 downregulation by CD4+ T cells from PN allergic children responding to PN allergens. Canonical discriminant analyses segregated non-allergic and allergic children by their cytokine secretion patterns, revealing differences and areas of overlap between PN and CME allergies. Despite an absence of recent allergen exposure, indication of in vivo activation, in vitro responses independent of challenging antigen and the presence of unusual costimulatory molecules suggest dysregulated immunity in food allergy. Most importantly, higher Bcl-2 content within key effector cells implies survival advantage with the potential to mount abnormal responses that may give rise to the manifestations of allergy. Here, we put forward the hypothesis that the lack of apoptosis of key immune cell types might be central to the development of food allergic reactions.
Keywords: apoptosis; cytokines; food allergy; immunoregulation; pediatric allergy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kwon H.K., Lee C.G., So J.S., Chae C.S., Hwang J.S., Sahoo A., Nam J.H., Rhee J.H., Hwang K.C., Im S.H. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

