AhR, PXR and CAR: From Xenobiotic Receptors to Metabolic Sensors
- PMID: 38067179
- PMCID: PMC10705969
- DOI: 10.3390/cells12232752
AhR, PXR and CAR: From Xenobiotic Receptors to Metabolic Sensors
Abstract
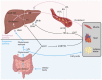
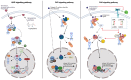
Traditionally, xenobiotic receptors are known for their role in chemical sensing and detoxification, as receptor activation regulates the expression of various key enzymes and receptors. However, recent studies have highlighted that xenobiotic receptors also play a key role in the regulation of lipid metabolism and therefore function also as metabolic sensors. Since dyslipidemia is a major risk factor for various cardiometabolic diseases, like atherosclerosis and non-alcoholic fatty liver disease, it is of major importance to understand the molecular mechanisms that are regulated by xenobiotic receptors. In this review, three major xenobiotic receptors will be discussed, being the aryl hydrocarbon receptor (AhR), pregnane X receptor (PXR) and the constitutive androstane receptor (CAR). Specifically, this review will focus on recent insights into the metabolic functions of these receptors, especially in the field of lipid metabolism and the associated dyslipidemia.
Keywords: aryl hydrocarbon receptor; cardiometabolic diseases; constitutive androstane receptor; lipid metabolism; pregnane X receptor; xenobiotic receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR.Adv Drug Deliv Rev. 2010 Oct 30;62(13):1238-49. doi: 10.1016/j.addr.2010.08.006. Epub 2010 Aug 17. Adv Drug Deliv Rev. 2010. PMID: 20727377 Free PMC article. Review.
-
Roles of xenobiotic receptors in vascular pathophysiology.Circ J. 2014;78(7):1520-30. doi: 10.1253/circj.cj-14-0343. Epub 2014 May 26. Circ J. 2014. PMID: 24859622 Review.
-
Regulation of Drug Metabolism by the Interplay of Inflammatory Signaling, Steatosis, and Xeno-Sensing Receptors in HepaRG Cells.Drug Metab Dispos. 2018 Apr;46(4):326-335. doi: 10.1124/dmd.117.078675. Epub 2018 Jan 12. Drug Metab Dispos. 2018. PMID: 29330220
-
The xenobiotic receptors PXR and CAR in liver physiology, an update.Biochim Biophys Acta Mol Basis Dis. 2021 Jun 1;1867(6):166101. doi: 10.1016/j.bbadis.2021.166101. Epub 2021 Feb 15. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33600998 Free PMC article. Review.
-
Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification.Mol Pharmacol. 2002 Sep;62(3):638-46. doi: 10.1124/mol.62.3.638. Mol Pharmacol. 2002. PMID: 12181440
Cited by
-
Relevance of kinetic interactions and co-formulants for plant protection product liver toxicity in vitro.Arch Toxicol. 2025 Aug;99(8):3247-3268. doi: 10.1007/s00204-025-04071-7. Epub 2025 Apr 28. Arch Toxicol. 2025. PMID: 40295322
-
Tuberculosis-diabetes comorbidities: Mechanistic insights for clinical considerations and treatment challenges.World J Diabetes. 2024 May 15;15(5):853-866. doi: 10.4239/wjd.v15.i5.853. World J Diabetes. 2024. PMID: 38766427 Free PMC article. Review.
-
Bile acids and their receptors in hepatic immunity.Liver Res. 2025 Jan 28;9(1):1-16. doi: 10.1016/j.livres.2025.01.005. eCollection 2025 Mar. Liver Res. 2025. PMID: 40206435 Free PMC article. Review.
-
Steatotic liver disease induced by TCPOBOP-activated hepatic constitutive androstane receptor: primary and secondary gene responses with links to disease progression.Toxicol Sci. 2024 Aug 1;200(2):324-345. doi: 10.1093/toxsci/kfae057. Toxicol Sci. 2024. PMID: 38710495 Free PMC article.
-
Induction of cytochrome P450 via upregulation of CAR and PXR: a potential mechanism for altered florfenicol metabolism by macranthoidin B in vivo.Front Pharmacol. 2024 Oct 9;15:1460948. doi: 10.3389/fphar.2024.1460948. eCollection 2024. Front Pharmacol. 2024. PMID: 39444610 Free PMC article.
References
-
- Hedayatnia M., Asadi Z., Zare-Feyzabadi R., Yaghooti-Khorasani M., Ghazizadeh H., Ghaffarian-Zirak R., Nosrati-Tirkani A., Mohammadi-Bajgiran M., Rohban M., Sadabadi F., et al. Dyslipidemia and Cardiovascular Disease Risk among the MASHAD Study Population. Lipids Health Dis. 2020;19:42. doi: 10.1186/s12944-020-01204-y. - DOI - PMC - PubMed
-
- Enjoji M., Kohjima M., Nakamuta M. Lipid Metabolism and the Liver. In: Ohira H., editor. The Liver in Systemic Diseases. Springer; Tokyo, Japan: 2016. pp. 105–122.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

