Targeting Striatal Glutamate and Phosphodiesterases to Control L-DOPA-Induced Dyskinesia
- PMID: 38067182
- PMCID: PMC10706484
- DOI: 10.3390/cells12232754
Targeting Striatal Glutamate and Phosphodiesterases to Control L-DOPA-Induced Dyskinesia
Abstract
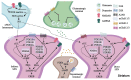
A large body of work during the past several decades has been focused on therapeutic strategies to control L-DOPA-induced dyskinesias (LIDs), common motor complications of long-term L-DOPA therapy in Parkinson's disease (PD). Yet, LIDs remain a clinical challenge for the management of patients with advanced disease. Glutamatergic dysregulation of striatal projection neurons (SPNs) appears to be a key contributor to altered motor responses to L-DOPA. Targeting striatal hyperactivity at the glutamatergic neurotransmission level led to significant preclinical and clinical trials of a variety of antiglutamatergic agents. In fact, the only FDA-approved treatment for LIDs is amantadine, a drug with NMDAR antagonistic actions. Still, novel agents with improved pharmacological profiles are needed for LID therapy. Recently other therapeutic targets to reduce dysregulated SPN activity at the signal transduction level have emerged. In particular, mechanisms regulating the levels of cyclic nucleotides play a major role in the transduction of dopamine signals in SPNs. The phosphodiesterases (PDEs), a large family of enzymes that degrade cyclic nucleotides in a specific manner, are of special interest. We will review the research for antiglutamatergic and PDE inhibition strategies in view of the future development of novel LID therapies.
Keywords: L-DOPA-induced dyskinesia; NMDAR; PDE10A; Parkinson’s disease; glutamate; phosphodiesterase; striatal projection neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Selective Phosphodiesterase 10A Inhibitor Reduces L-Dopa-Induced Dyskinesias in Parkinsonian Monkeys.Mov Disord. 2018 May;33(5):805-814. doi: 10.1002/mds.27341. Epub 2018 Mar 6. Mov Disord. 2018. PMID: 29508924 Free PMC article.
-
Chronic H3R activation reduces L-Dopa-induced dyskinesia, normalizes cortical GABA and glutamate levels, and increases striatal dopamine D1R mRNA expression in 6-hydroxydopamine-lesioned male rats.Psychopharmacology (Berl). 2023 Jun;240(6):1221-1234. doi: 10.1007/s00213-023-06339-1. Epub 2023 Apr 22. Psychopharmacology (Berl). 2023. PMID: 37086286
-
Ameliorative effects of a phosphodiesterase 10A inhibitor, MR1916 on L-DOPA-induced dyskinesia in parkinsonian rats.Pharmacol Rep. 2020 Apr;72(2):443-448. doi: 10.1007/s43440-020-00060-y. Epub 2020 Mar 6. Pharmacol Rep. 2020. PMID: 32144743
-
Efficacy and safety of amantadine for the treatment of L-DOPA-induced dyskinesia.J Neural Transm (Vienna). 2018 Aug;125(8):1237-1250. doi: 10.1007/s00702-018-1869-1. Epub 2018 Mar 7. J Neural Transm (Vienna). 2018. PMID: 29511826 Review.
-
Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson's disease.CNS Drugs. 2014 Dec;28(12):1155-84. doi: 10.1007/s40263-014-0205-z. CNS Drugs. 2014. PMID: 25342080 Review.
Cited by
-
The Effect of Chronic Treatment with the Inhibitor of Phosphodiesterase 5 (PDE5), Sildenafil, in Combination with L-DOPA on Asymmetric Behavior and Monoamine Catabolism in the Striatum and Substantia Nigra of Unilaterally 6-OHDA-Lesioned Rats.Molecules. 2024 Sep 11;29(18):4318. doi: 10.3390/molecules29184318. Molecules. 2024. PMID: 39339313 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

