The Molecular Mechanisms Responsible for Tear Hyperosmolarity-Induced Pathological Changes in the Eyes of Dry Eye Disease Patients
- PMID: 38067183
- PMCID: PMC10706334
- DOI: 10.3390/cells12232755
The Molecular Mechanisms Responsible for Tear Hyperosmolarity-Induced Pathological Changes in the Eyes of Dry Eye Disease Patients
Abstract
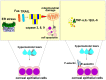
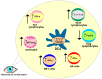
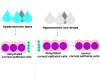
Dry eye disease (DED) is a multifactorial disorder of the lacrimal system and ocular surface, characterized by a deficiency in the quality and/or quantity of the tear fluid. The multifactorial nature of DED encompasses a number of interconnected underlying pathologies, including loss of homeostasis, instability and hyperosmolarity of the tears, and the induction and propagation of detrimental inflammatory responses in the eyes, which finally results in the development of neurosensory dysfunction and visual disruption. Dryness, grittiness, scratchiness, discomfort, inflammation, burning, watering, ocular fatigue, pain, and decreased functional visual acuity are common symptoms of DED. Eye dysfunction drastically attenuates patients' quality of life. Accordingly, a better understanding of the pathogenic processes that regulate the development and progression of DED is crucially important for the establishment of new and more effective DED-related treatment approaches, which would significantly improve the quality of life of DED patients. Since the process of osmoregulation, which guards the ocular surface epithelia and maintains normal vision, is affected when the osmolarity of the tears is greater than that of the epithelial cells, tear hyperosmolarity (THO) is considered an initial, important step in the development, progression, and aggravation of DED. In order to delineate the role of THO in the pathogenesis of DED, in this review article, we summarize current knowledge related to the molecular mechanisms responsible for the development of THO-induced pathological changes in the eyes of DED patients, and we briefly discuss the therapeutic potential of hypo-osmotic eye drops in DED treatment.
Keywords: corneal epithelial cells; dry eye disease; eye inflammation; hypo-osmotic eye drops; tear hyperosmolarity.
Conflict of interest statement
Author C.R.H. was employed by the company Regenerative Processing Plant. The remaining authors declare no conflict of interest.
Figures

References
-
- Milner M.S., Beckman K.A., Luchs J.I., Allen Q.B., Awdeh R.M., Berdahl J., Boland T.S., Buznego C., Gira J.P., Goldberg D.F., et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders—New strategies for diagnosis and treatment. Curr. Opin. Ophthalmol. 2017;27((Suppl. S1)):3–47. doi: 10.1097/01.icu.0000512373.81749.b7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

