Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool
- PMID: 38067189
- PMCID: PMC10705965
- DOI: 10.3390/cells12232761
Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool
Abstract
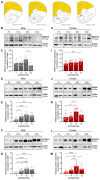
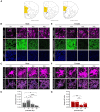
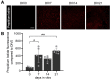
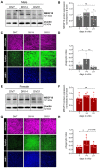
Clinical and pre-clinical studies of neuropsychiatric (NP) disorders show altered astrocyte properties and synaptic networks. These are refined during early postnatal developmental (PND) stages. Thus, investigating early brain maturational trajectories is essential to understand NP disorders. However, animal experiments are highly time-/resource-consuming, thereby calling for alternative methodological approaches. The function of MEGF10 in astrocyte-mediated synapse elimination (pruning) is crucial to refine neuronal networks during development and adulthood. To investigate the impact of MEGF10 during PND in the rat prefrontal cortex (PFC) and its putative role in brain disorders, we established and validated an organotypic brain slice culture (OBSC) system. Using Western blot, we characterized the expression of MEGF10 and the synaptic markers synaptophysin and PSD95 in the cortex of developing pups. We then combined immunofluorescent-immunohistochemistry with Imaris-supported 3D analysis to compare age- and sex-dependent astrocyte-mediated pruning within the PFC in pups and OBSCs. We thereby validated this system to investigate age-dependent astrocyte-mediated changes in pruning during PND. However, further optimizations are required to use OBSCs for revealing sex-dependent differences. In conclusion, OBSCs offer a valid alternative to study physiological astrocyte-mediated synaptic remodeling during PND and might be exploited to investigate the pathomechanisms of brain disorders with aberrant synaptic development.
Keywords: astrocyte; critical period; organotypic brain slice culture; sex differences; synaptic phagocytosis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Cortical Organotypic Brain Slice Cultures to Examine Sex- and Age-Dependent Astrocyte-Mediated Synaptic Phagocytosis.Methods Mol Biol. 2025;2896:203-214. doi: 10.1007/978-1-0716-4366-2_15. Methods Mol Biol. 2025. PMID: 40111607
-
Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats.Pharmacol Res. 2016 Sep;111:459-470. doi: 10.1016/j.phrs.2016.07.008. Epub 2016 Jul 12. Pharmacol Res. 2016. PMID: 27422357
-
Diverging Effects of Adolescent Ethanol Exposure on Tripartite Synaptic Development across Prefrontal Cortex Subregions.Cells. 2022 Oct 2;11(19):3111. doi: 10.3390/cells11193111. Cells. 2022. PMID: 36231073 Free PMC article.
-
Brain stars take the lead during critical periods of early postnatal brain development: relevance of astrocytes in health and mental disorders.Mol Psychiatry. 2024 Sep;29(9):2821-2833. doi: 10.1038/s41380-024-02534-4. Epub 2024 Mar 29. Mol Psychiatry. 2024. PMID: 38553540 Free PMC article. Review.
-
Astrocyte-dependent circuit remodeling by synapse phagocytosis.Curr Opin Neurobiol. 2023 Aug;81:102732. doi: 10.1016/j.conb.2023.102732. Epub 2023 May 27. Curr Opin Neurobiol. 2023. PMID: 37247606 Review.
Cited by
-
Cortical Organotypic Brain Slice Cultures to Examine Sex- and Age-Dependent Astrocyte-Mediated Synaptic Phagocytosis.Methods Mol Biol. 2025;2896:203-214. doi: 10.1007/978-1-0716-4366-2_15. Methods Mol Biol. 2025. PMID: 40111607
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

