Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function
- PMID: 38067192
- PMCID: PMC10705885
- DOI: 10.3390/cells12232764
Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function
Abstract
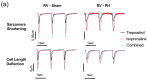
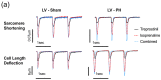
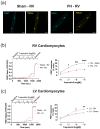
(1) Pulmonary hypertension (PH)-associated right ventricular (RV) failure is linked to a reduction in pulmonary vasodilators. Treprostinil has shown effectiveness in PAH patients with cardiac decompensation, hinting at potential cardiac benefits. We investigated treprostinil's synergy with isoprenaline in RV and LV cardiomyocytes. We hypothesised that disease-related RV structural changes in cardiomyocytes would reduce contractile responses and cAMP/PKA signalling activity. (2) We induced PH in male Sprague Dawley rats using monocrotaline and isolated their ventricular cardiomyocytes. The effect of in vitro treprostinil and isoprenaline stimulation on contraction was assessed. FRET microscopy was used to study PKA activity associated with treprostinil stimulation in AKAR3-NES FRET-based biosensor-expressing cells. (3) RV cells exhibited maladaptive remodelling with hypertrophy, impaired contractility, and calcium transients compared to control and LV cardiomyocytes. Combining treprostinil and isoprenaline failed to enhance inotropy in PH RV cardiomyocytes. PH RV cardiomyocytes displayed an aberrant contractile behaviour, which the combination treatment could not rectify. Finally, we observed decreased PKA activity in treprostinil-treated PH RV cardiomyocytes. (4) PH-associated RV cardiomyocyte remodelling reduced treprostinil sensitivity, inotropic support, and impaired relaxation. Overall, this study highlights the complexity of RV dysfunction in advanced PH and suggests the need for alternative therapeutic strategies.
Keywords: cardiomyocytes; cell length deflection; pulmonary hypertension; right ventricle; sarcomere shortening; treprostinil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Geraci M.W., Gao B., Shepherd D.C., Moore M.D., Westcott J.Y., Fagan K.A., Alger L.A., Tuder R.M., Voelkel N.F. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J. Clin. Investig. 1999;103:1509–1515. doi: 10.1172/JCI5911. - DOI - PMC - PubMed
-
- Handoko M.L., de Man F.S., Allaart C.P., Paulus W.J., Westerhof N., Vonk-Noordegraaf A. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: Lessons from the left heart. Eur. Respir. Rev. 2010;19:72–82. doi: 10.1183/09059180.00007109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

