Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications
- PMID: 38067195
- PMCID: PMC10706122
- DOI: 10.3390/cells12232767
Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications
Abstract
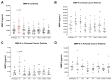
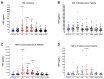
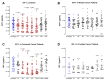
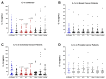
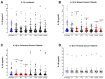
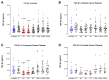
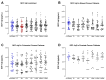
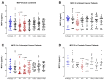
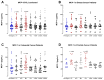
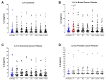
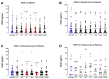
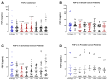
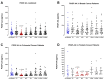
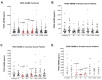
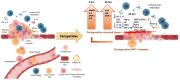
Surgery-induced tumor growth acceleration and synchronous metastatic growth promotion have been observed for decades. Surgery-induced wound healing, orchestrated through growth factors, chemokines, and cytokines, can negatively impact patients harboring residual or metastatic disease. We provide detailed clinical evidence of this process in surgical breast, prostate, and colorectal cancer patients. Plasma samples were analyzed from 68 cancer patients who had not received treatment before surgery or adjuvant therapy until at least four weeks post-surgery. The levels of plasma cytokines, chemokines, and growth factors were simultaneously quantified and profiled using multiplexed immunoassays for eight time points sampled per patient. The immunologic processes are induced immediately after surgery in patients, characterized by a drastic short-term shift in the expression levels of pro-inflammatory and angiogenic molecules and cytokines. A rapid and significant spike in circulating plasma levels of hepatocyte growth factor (HGF), interleukin-6 (IL-6), placental growth factor (PLGF), and matrix metalloproteinase-9 (MMP-9) after surgery was noted. The rise in these molecules was concomitant with a significant drop in transforming growth factor-β1 (TGF-β1), platelet-derived growth factor (PDGF-AB/BB), insulin-like growth factor-1 (IGF-1), and monocyte chemoattractant protein-2 (MCP-2). If not earlier, each plasma analyte was normalized to baseline levels within 1-2 weeks after surgery, suggesting that surgical intervention alone was responsible for these effects. The effects of surgical tumor removal on disrupting the pro-inflammatory and angiogenic plasma profiles of cancer patients provide evidence for potentiating malignant progression. Our findings indicate a narrow therapeutic window of opportunity after surgery to prevent disease recurrence.
Keywords: chemokines; colorectal cancer patients; cytokines; growth factors; pro-inflammatory and angiogenic plasma profiles of cancer patients; prostate; surgery-induced wound healing; surgical breast.
Conflict of interest statement
William W. Harless owns shares in ENCYT and has commercial interest and/or patents in the work under consideration. Alexander Wawer and Beth Lewis own shares in ENCYT.
Figures





Similar articles
-
Identification of colorectal cancer metastasis markers by an angiogenesis-related cytokine-antibody array.World J Gastroenterol. 2012 Feb 21;18(7):637-45. doi: 10.3748/wjg.v18.i7.637. World J Gastroenterol. 2012. PMID: 22363134 Free PMC article.
-
Pilot study of angiogenic response to yttrium-90 radioembolization with resin microspheres.J Vasc Interv Radiol. 2014 Feb;25(2):297-306.e1. doi: 10.1016/j.jvir.2013.10.030. Epub 2013 Dec 20. J Vasc Interv Radiol. 2014. PMID: 24360887 Free PMC article.
-
Cytokine and Chemokine Concentration in the Tear of Patients with Age-Related Cataract.Curr Eye Res. 2020 Sep;45(9):1101-1106. doi: 10.1080/02713683.2020.1715445. Epub 2020 Jan 24. Curr Eye Res. 2020. PMID: 31928443
-
Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth.Prog Biophys Mol Biol. 2013 Nov;113(2):333-54. doi: 10.1016/j.pbiomolbio.2013.10.001. Epub 2013 Oct 15. Prog Biophys Mol Biol. 2013. PMID: 24139944 Review.
-
Perioperative host-tumor inflammatory interactions: a potential trigger for disease recurrence following a curative resection for colorectal cancer.Surg Today. 2008;38(7):579-84. doi: 10.1007/s00595-007-3674-6. Epub 2008 Jul 9. Surg Today. 2008. PMID: 18612780 Review.
Cited by
-
Prostate cancer microenvironment: multidimensional regulation of immune cells, vascular system, stromal cells, and microbiota.Mol Cancer. 2024 Oct 12;23(1):229. doi: 10.1186/s12943-024-02137-1. Mol Cancer. 2024. PMID: 39395984 Free PMC article. Review.
-
Pro-Inflammatory Cytokines Transactivate Glycosylated Cytokine Receptors on Cancer Cells to Induce Epithelial-Mesenchymal Transition to the Metastatic Phenotype.Cancers (Basel). 2025 Apr 5;17(7):1234. doi: 10.3390/cancers17071234. Cancers (Basel). 2025. PMID: 40227834 Free PMC article.
-
Contemporaneous Inflammatory, Angiogenic, Fibrogenic, and Angiostatic Cytokine Profiles of the Time-to-Tumor Development by Cancer Cells to Orchestrate Tumor Neovascularization, Progression, and Metastasis.Cells. 2024 Oct 20;13(20):1739. doi: 10.3390/cells13201739. Cells. 2024. PMID: 39451257 Free PMC article.
-
Comparison of postoperative analgesia between dezocine plus flurbiprofen axetil and sufentanil in patients with CRC undergoing tumor resection: A prospective, observational study.Oncol Lett. 2025 Jan 7;29(3):121. doi: 10.3892/ol.2025.14869. eCollection 2025 Mar. Oncol Lett. 2025. PMID: 39807102 Free PMC article.
-
Perioperative Clinical Usage of Phellinus Linteus as a Nutraceutical for Non-FOLFIRINOX-Based Postoperative Adjuvant Chemotherapy for Resected Pancreatic Cancer: A Retrospective Cohort Study.Integr Cancer Ther. 2025 Jan-Dec;24:15347354251353499. doi: 10.1177/15347354251353499. Epub 2025 Jul 1. Integr Cancer Ther. 2025. PMID: 40590265 Free PMC article.
References
-
- Yamashita J.I., Kurusu Y., Fujino N., Saisyoji T., Ogawa M. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: A potential hazard for intraoperative hematogenous tumor cell dissemination. J. Thorac. Cardiovasc. Surg. 2000;119:899–905. doi: 10.1016/S0022-5223(00)70084-5. - DOI - PubMed
-
- Ge M.J., Shi D., Wu Q.C., Wang M., Li L.B. Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J. Cancer Res. Clin. Oncol. 2006;132:248–256. doi: 10.1007/s00432-005-0059-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

