Neoadjuvant Approaches to Non-Melanoma Skin Cancer
- PMID: 38067198
- PMCID: PMC10705727
- DOI: 10.3390/cancers15235494
Neoadjuvant Approaches to Non-Melanoma Skin Cancer
Abstract
Surgery and external-beam radiation therapy are the primary treatment modalities for locally advanced NMSC, but they can lead to impairment of function and disfigurement in sensitive areas such as the head and neck. With the advent of targeted systemic therapies and immunotherapy, physicians have explored the ability to offer neoadjuvant therapy for NMSC in order to reduce surgically induced morbidity. Provided herein is a guide to current applications of neoadjuvant systemic therapies for NMSC and future directions.
Keywords: Merkel cell carcinoma; basal cell carcinoma; cutaneous squamous cell carcinoma; neoadjuvant therapy; non-melanomatous skin cancer.
Conflict of interest statement
M.K.K.W. has served on advisory boards for the following: Merck, Pfizer, Bristol Myers Squibb, Regeneron, EMD-Serono, ExiCure, Castle Biosciences, Replimune, and Incyte. N.D.G. reports institutional research funding from Regeneron Pharmaceuticals, Inc., and advisory board and consulting fees from PDS Biotechnology, Replimmune, and Merck.
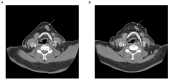
Figures

Similar articles
-
Surgery in the Era of Immunotherapy for Advanced Head and Neck Non-melanoma Skin Cancer.Curr Oncol Rep. 2023 Jul;25(7):735-742. doi: 10.1007/s11912-023-01391-8. Epub 2023 Apr 3. Curr Oncol Rep. 2023. PMID: 37010785 Review.
-
Neoadjuvant Therapy for Non-melanoma Skin Cancer: Updated Therapeutic Approaches for Basal, Squamous, and Merkel Cell Carcinoma.Curr Treat Options Oncol. 2021 Mar 16;22(4):35. doi: 10.1007/s11864-021-00826-3. Curr Treat Options Oncol. 2021. PMID: 33725197 Free PMC article. Review.
-
The Role of Radiation Therapy in the Treatment of Non-Melanoma Skin Cancer.Cancers (Basel). 2023 Apr 22;15(9):2408. doi: 10.3390/cancers15092408. Cancers (Basel). 2023. PMID: 37173875 Free PMC article. Review.
-
Evolving Role of Systemic Therapies in Non-melanoma Skin Cancer.Clin Oncol (R Coll Radiol). 2019 Nov;31(11):759-768. doi: 10.1016/j.clon.2019.08.011. Epub 2019 Sep 12. Clin Oncol (R Coll Radiol). 2019. PMID: 31522944 Review.
-
Immunotherapy for Non-melanoma Skin Cancer.Curr Oncol Rep. 2021 Aug 27;23(11):125. doi: 10.1007/s11912-021-01120-z. Curr Oncol Rep. 2021. PMID: 34448958 Free PMC article. Review.
Cited by
-
The rapidly evolving paradigm of neoadjuvant immunotherapy across cancer types.Nat Cancer. 2025 Jun;6(6):967-987. doi: 10.1038/s43018-025-00990-7. Epub 2025 Jun 24. Nat Cancer. 2025. PMID: 40555863 Review.
-
Neoadjuvant Cetuximab Leading to a Complete Pathologic Response in Locally Advanced Lip Squamous Cell Carcinoma.Cureus. 2024 Dec 2;16(12):e75000. doi: 10.7759/cureus.75000. eCollection 2024 Dec. Cureus. 2024. PMID: 39749049 Free PMC article.
-
Oncolytic viral therapy for nonmelanoma skin cancer and cutaneous lymphoma - A systematic review.JAAD Int. 2025 Feb 7;20:4-20. doi: 10.1016/j.jdin.2024.11.010. eCollection 2025 Jun. JAAD Int. 2025. PMID: 40213531 Free PMC article. Review.
-
Advanced Basal Cell Carcinoma: A Narrative Review on Current Systemic Treatments and the Neoadjuvant Approach.J Pers Med. 2025 Jun 1;15(6):226. doi: 10.3390/jpm15060226. J Pers Med. 2025. PMID: 40559089 Free PMC article. Review.
-
Natural Compounds in Non-Melanoma Skin Cancer: Prevention and Treatment.Molecules. 2024 Feb 4;29(3):728. doi: 10.3390/molecules29030728. Molecules. 2024. PMID: 38338469 Free PMC article. Review.
References
-
- Society A.C. American Cancer Society Facts and Figures 2022. 2022. [(accessed on 2 July 2023)]. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-....
-
- Topalian S.L., Bhatia S., Amin A., Kudchadkar R.R., Sharfman W.H., Lebbe C., Delord J.P., Dunn L.A., Shinohara M.M., Kulikauskas R., et al. Neoadjuvant Nivolumab for Patients with Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020;38:2476–2487. doi: 10.1200/JCO.20.00201. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

