Regulation of Vascular Endothelial Growth Factor Signaling by Nicotine in a Manner Dependent on Acetylcholine-and/or β-Adrenergic-Receptors in Human Lung Cancer Cells
- PMID: 38067204
- PMCID: PMC10705358
- DOI: 10.3390/cancers15235500
Regulation of Vascular Endothelial Growth Factor Signaling by Nicotine in a Manner Dependent on Acetylcholine-and/or β-Adrenergic-Receptors in Human Lung Cancer Cells
Abstract
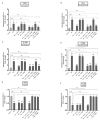
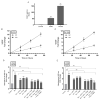
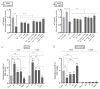
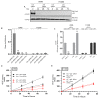
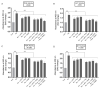
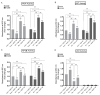
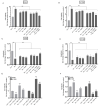
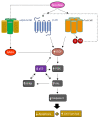
In addition to binding to nicotinic acetylcholine receptors (nAChRs), nicotine is known to regulate the β-adrenergic receptors (β-ARs) promoting oncogenic signaling. Using A549 (p53 wild-type) and H1299 (p53-null) lung cancer cells, we show that nicotine treatment led to: increased adrenaline/noradrenaline levels, an effect blocked by treatment with the α7nAChR inhibitor (α-BTX) but not by the β-blocker (propranolol) or the α4β2nAChR antagonist (DhβE); decreased GABA levels in A549 and H1299 cell media, an effect blocked by treatment with DhβE; increased VEGF levels and PI3K/AKT activities, an effect diminished by cell co-treatment with α-BTX, propranolol, and/or DhβE; and inhibited p53 activity in A549 cells, that was reversed, upon cell co-treatment with α-BTX, propranolol, and/or DhβE or by VEGF immunodepletion. VEGF levels increased upon cell treatment with nicotine, adrenaline/noradrenaline, and decreased with GABA treatment. On the other hand, the p53 activity decreased in A549 cells treated with nicotine, adrenaline/noradrenaline and increased upon cell incubation with GABA. Knockdown of p53 led to increased VEGF levels in the media of A549 cells. The addition of anti-VEGF antibodies to A549 and H1299 cells decreased cell viability and increased apoptosis; blocked the activities of PI3K, AKT, and NFκB in the absence or presence of nicotine; and resulted in increased p53 activation in A549 cells. We conclude that VEGF can be upregulated via α7nAChR and/or β-ARs and downregulated via GABA and/or p53 in response to the nicotine treatment of NSCLC cells.
Keywords: AKT; GABA; NFκB; PI3K; lung cancer; nicotine; nicotinic acetylcholine receptors; p53; vascular endothelial growth factor; β-adrenergic receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention.PLoS One. 2012;7(1):e29915. doi: 10.1371/journal.pone.0029915. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253823 Free PMC article.
-
Regulation of Soluble E-Cadherin Signaling in Non-Small-Cell Lung Cancer Cells by Nicotine, BDNF, and β-Adrenergic Receptor Ligands.Biomedicines. 2023 Sep 18;11(9):2555. doi: 10.3390/biomedicines11092555. Biomedicines. 2023. PMID: 37760996 Free PMC article.
-
Regulation of Cisplatin Resistance in Lung Cancer Cells by Nicotine, BDNF, and a β-Adrenergic Receptor Blocker.Int J Mol Sci. 2022 Oct 24;23(21):12829. doi: 10.3390/ijms232112829. Int J Mol Sci. 2022. PMID: 36361620 Free PMC article.
-
Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways.Toxicol Appl Pharmacol. 2008 Dec 1;233(2):254-61. doi: 10.1016/j.taap.2008.08.012. Epub 2008 Sep 6. Toxicol Appl Pharmacol. 2008. PMID: 18805435
-
Gamma-amino butyric acid inhibits the nicotine-imposed stimulatory challenge in xenograft models of non-small cell lung carcinoma.Curr Cancer Drug Targets. 2012 Feb;12(2):97-106. doi: 10.2174/156800912799095171. Curr Cancer Drug Targets. 2012. PMID: 22165966 Review.
Cited by
-
The role of leptin in regulation of the soluble amyloid precursor protein α (sAPPα) levels in lung cancer cell media.Sci Rep. 2024 Feb 28;14(1):4921. doi: 10.1038/s41598-024-55717-y. Sci Rep. 2024. PMID: 38418632 Free PMC article.
-
Mechanisms of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (huang qi) and Angelica sinensis (Oliv.) Diels (dang gui) in Ameliorating Hypoxia and Angiogenesis to Delay Pulmonary Nodule Malignant Transformation.Integr Cancer Ther. 2025 Jan-Dec;24:15347354241311917. doi: 10.1177/15347354241311917. Integr Cancer Ther. 2025. PMID: 39882753 Free PMC article. Review.
-
Amyloid Beta Leads to Decreased Acetylcholine Levels and Non-Small Cell Lung Cancer Cell Survival via a Mechanism That Involves p38 Mitogen-Activated Protein Kinase and Protein Kinase C in a p53-Dependent and -Independent Manner.Int J Mol Sci. 2024 May 5;25(9):5033. doi: 10.3390/ijms25095033. Int J Mol Sci. 2024. PMID: 38732252 Free PMC article.
-
The interplay between gut bacteria and targeted therapies: implications for future cancer treatments.Mol Med. 2025 Feb 13;31(1):58. doi: 10.1186/s10020-025-01108-6. Mol Med. 2025. PMID: 39948481 Free PMC article. Review.
References
-
- Siddiqui F., Siddiqui A.H. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Lung Cancer.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

