Advances of Protein Palmitoylation in Tumor Cell Deaths
- PMID: 38067206
- PMCID: PMC10705081
- DOI: 10.3390/cancers15235503
Advances of Protein Palmitoylation in Tumor Cell Deaths
Abstract
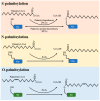
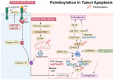
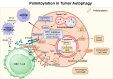
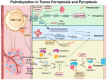
In this comprehensive survey, we delve into the multifaceted role of palmitoylation across various cell death modalities in the oncological context, from its intricate correlations with tumorigenesis, steered by the Asp-His-His-Cys tetrapeptide motif (DHHC) family, to the counter-process of depalmitoylation mediated by enzymes like Palmitoyl protein thioesterase-1 (PPT1). Innovations in detection methodologies have paralleled our growing understanding, transitioning from rudimentary techniques to sophisticated modern methods. Central to our discourse are agents like Ezurpimtrostat (GNS561) and dimeric chloroquine (DC661), promising heralds in palmitoylation-targeted cancer therapy. Collectively, this review accentuates palmitoylation's transformative potential in oncology, foreshadowing groundbreaking therapeutic strategies and deepening our molecular comprehension of cancer dynamics.
Keywords: apoptosis; autophagy; ferroptosis; protein palmitoylation; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Dual Regulation of Sprouty 4 Palmitoylation by ZDHHC7 and Palmitoyl-Protein Thioesterase 1: A Potential Therapeutic Strategy for Cisplatin-Resistant Osteosarcoma.Research (Wash D C). 2025 May 23;8:0708. doi: 10.34133/research.0708. eCollection 2025. Research (Wash D C). 2025. PMID: 40416361 Free PMC article.
-
Depalmitoylation by Palmitoyl-Protein Thioesterase 1 in Neuronal Health and Degeneration.Front Synaptic Neurosci. 2019 Aug 29;11:25. doi: 10.3389/fnsyn.2019.00025. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31555119 Free PMC article. Review.
-
First-In-Human Effects of PPT1 Inhibition Using the Oral Treatment with GNS561/Ezurpimtrostat in Patients with Primary and Secondary Liver Cancers.Liver Cancer. 2022 Feb 15;11(3):268-277. doi: 10.1159/000522418. eCollection 2022 Jun. Liver Cancer. 2022. PMID: 35949290 Free PMC article.
-
Protein palmitoylation in cancer: molecular functions and therapeutic potential.Mol Oncol. 2023 Jan;17(1):3-26. doi: 10.1002/1878-0261.13308. Epub 2022 Sep 10. Mol Oncol. 2023. PMID: 36018061 Free PMC article. Review.
-
Emerging roles for protein S-palmitoylation in Toxoplasma biology.Int J Parasitol. 2014 Feb;44(2):121-31. doi: 10.1016/j.ijpara.2013.09.004. Epub 2013 Nov 1. Int J Parasitol. 2014. PMID: 24184909 Review.
Cited by
-
Research trends of protein palmitoylation in cancer from 2004 to 2024: a bibliometric and visualization analysis.Front Oncol. 2025 Jun 23;15:1571870. doi: 10.3389/fonc.2025.1571870. eCollection 2025. Front Oncol. 2025. PMID: 40626019 Free PMC article.
-
Dual Regulation of Sprouty 4 Palmitoylation by ZDHHC7 and Palmitoyl-Protein Thioesterase 1: A Potential Therapeutic Strategy for Cisplatin-Resistant Osteosarcoma.Research (Wash D C). 2025 May 23;8:0708. doi: 10.34133/research.0708. eCollection 2025. Research (Wash D C). 2025. PMID: 40416361 Free PMC article.
-
[Research Progress and Applications of ZDHHC-mediated Protein Palmitoylation in the Development and Immune Escape of Non-small Cell Lung Cancer].Zhongguo Fei Ai Za Zhi. 2025 Apr 20;28(4):319-324. doi: 10.3779/j.issn.1009-3419.2025.102.15. Zhongguo Fei Ai Za Zhi. 2025. PMID: 40404480 Free PMC article. Review. Chinese.
-
Regulation of pattern recognition receptor signaling by palmitoylation.iScience. 2024 Dec 20;28(2):111667. doi: 10.1016/j.isci.2024.111667. eCollection 2025 Feb 21. iScience. 2024. PMID: 39877903 Free PMC article. Review.
-
Palmitoyltransferase ZDHHC6 promotes colon tumorigenesis by targeting PPARγ-driven lipid biosynthesis via regulating lipidome metabolic reprogramming.J Exp Clin Cancer Res. 2024 Aug 16;43(1):227. doi: 10.1186/s13046-024-03154-0. J Exp Clin Cancer Res. 2024. PMID: 39148124 Free PMC article.
References
-
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
Publication types
Grants and funding
- Pdjh2022c0098/the Special Funds for the Cultivation of Guangdong College Students Scientific and Technological Innovation
- 2022G19/the National Training Program of Undergraduate Innovation and Entrepreneurship
- 32070625/National Natural Science Foundation of China
- A2021436/the Medical science and technology research fund project of Guangdong Province
- JCYJ20210324104800001, JCYJ20220530114415036/Shenzhen Municipal Science and Technology Innovation Commission Foundation
LinkOut - more resources
Full Text Sources

