PARP1 Characterization as a Potential Biomarker for BCR::ABL1 p190+ Acute Lymphoblastic Leukemia
- PMID: 38067214
- PMCID: PMC10705790
- DOI: 10.3390/cancers15235510
PARP1 Characterization as a Potential Biomarker for BCR::ABL1 p190+ Acute Lymphoblastic Leukemia
Abstract
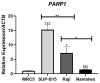
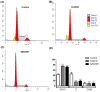
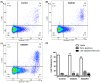
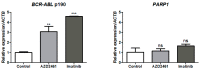
Detection of t(9;22), and consequent BCR::ABL1 fusion, is still a marker of worse prognosis for acute lymphoblastic leukemia (ALL), with resistance to tyrosine-kinase inhibitor therapy being a major obstacle in the clinical practice for this subset of patients. In this study, we investigated the effectiveness of targeting poly-ADP-ribose polymerase (PARP) in a model of BCR::ABL1 p190+ ALL, the most common isoform to afflict ALL patients, and demonstrated the use of experimental PARP inhibitor (PARPi), AZD2461, as a therapeutic option with cytotoxic capabilities similar to that of imatinib, the current gold standard in medical care. We characterized cytostatic profiles, induced cell death, and biomarker expression modulation utilizing cell models, also providing a comprehensive genome-wide analysis through an aCGH of the model used, and further validated PARP1 differential expression in samples of ALL p190+ patients from local healthcare institutions, as well as in larger cohorts of online and readily available datasets. Overall, we demonstrate the effectiveness of PARPi in the treatment of BCR::ABL1 p190+ ALL cell models and that PARP1 is differentially expressed in patient samples. We hope our findings help expand the characterization of molecular profiles in ALL settings and guide future investigations into novel biomarker detection and pharmacological choices in clinical practice.
Keywords: acute lymphoblastic leukemia; drug repositioning; molecular targeted therapy; poly(ADP-ribose) polymerase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
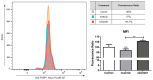


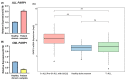

References
-
- Adnan-Awad S., Kim D., Hohtari H., Javarappa K.K., Brandstoetter T., Mayer I., Potdar S., Heckman C.A., Kytölä S., Porkka K., et al. Characterization of P190-Bcr-Abl Chronic Myeloid Leukemia Reveals Specific Signaling Pathways and Therapeutic Targets. Leukemia. 2021;35:1964. doi: 10.1038/s41375-020-01082-4. - DOI - PMC - PubMed
Grants and funding
- 88887.804627/2023-00/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 404213/2021-9/National Council for Scientific and Technological Development
- 101375/2022-0/National Council for Scientific and Technological Development
- 22/09926-2/São Paulo Research Foundation
- 2020/11413-8/São Paulo Research Foundation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

