Concordance between Three Homologous Recombination Deficiency (HRD) Assays in Patients with High-Grade Epithelial Ovarian Cancer
- PMID: 38067228
- PMCID: PMC10705222
- DOI: 10.3390/cancers15235525
Concordance between Three Homologous Recombination Deficiency (HRD) Assays in Patients with High-Grade Epithelial Ovarian Cancer
Abstract
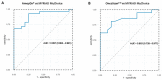
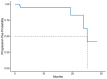
Our aim was to evaluate the concordance between the Myriad MyChoice and two alternative homologous recombination deficiency (HRD) assays (AmoyDx HRD Focus NGS Panel and OncoScan™) in patients with epithelial ovarian cancer (EOC). Tissue samples from 50 patients with newly diagnosed EOC and known Myriad MyChoice HRD status were included. DNA aliquots from tumor samples, previously evaluated with Myriad MyChoice and centrally reassessed, were distributed to laboratories to assess their HRD status using the two platforms, after being blinded for the Myriad MyChoice CDx HRD status. The primary endpoint was the concordance between Myriad MyChoice and each alternative assay. Tumor samples were evaluated with an AmoyDx® HRD Focus Panel (n = 50) and with OncoScan™ (n = 43). Both platforms provided results for all tumors. Analysis showed that correlation was high for the Myriad MyChoice GI score and AmoyDx® HRD Focus Panel (r = 0.79) or OncoScan™ (r = 0.87) (continuous variable). The overall percent agreement (OPA) between Myriad MyChoice GI status (categorical variable) and each alternative assay was 83.3% (68.6-93.3%) with AmoyDx and 77.5% (61.5-89.2%) with OncoScan™. The OPA in HRD status between Myriad MyChoice and AmoyDx was 88.6% (75.4-96.2). False-positive rates were 31.6% (6/19) for AmoyDx GI status and 31.9% (7/22) for OncoScan™, while false-negative rates were 0% (0/28, AmoyDx) and 11.1% (2/18, OncoScan™) compared with the Myriad MyChoice GI status. While substantial concordance between Myriad MyChoice and alternative assays was demonstrated, prospective validation of the analytical performance and clinical relevance of these assays is warranted.
Keywords: biomarker; concordance; enomic instability; epithelial ovarian cancer; homologous recombination deficiency.
Conflict of interest statement
E.F.: Advisory role: LEO Pharma. Speaker fees: Roche, Pfizer, AstraZeneca. Stock ownership: Genprex Inc., Deciphera Pharmaceuticals Inc. Travel grant: Merck, Pfizer, K.A.M Oncology/Hematology and DEMO. SK: Consultation/Advisory: Astra Zeneca, Roche. Research: Novartis, Roche. Speaker: Astra Zeneca, Novartis, Roche. A.K.: Advisory role: Genesis Pharma. Honoraria: Pfizer. Speaker’s bureau: Roche. Research funding: Merck. Travel: MSD. Educational grants: Novartis, Pfizer, Merck, Roche, BMS, MSD, Genesis and Ipsen. G.F.: Advisory Board: Pfizer, Novartis. Honoraria: AstraZeneca, Novartis. Stock ownership: Genprex Inc., Daiichi Sankyo, RFL Holdings, FORMYCON. M.L.: Employment: Pfizer. Stock and other ownership interests: Pfizer. Honoraria: Astellas Pharma, AstraZeneca, Bristol-Myers Squibb/Celgene, Ipsen, Janssen, MSD Oncology, Roche, Sanofi. Consulting or advisory role: Amgen, AstraZeneca, GlaxoSmithKline, Janssen. Travel accommodations, expenses: AstraZeneca, Bayer, Pfizer, Roche; (I): Immediate Family Member. The rest of the authors declare no conflict of interest.
Figures

References
-
- Arora T., Mullangi S., Lekkala M.R. Ovarian Cancer. StatPearls; Treasure Island, FL, USA: 2023. - PubMed
-
- Penson R.T., Valencia R.V., Cibula D., Colombo N., Leath C.A., 3rd, Bidzinski M., Kim J.W., Nam J.H., Madry R., Hernandez C., et al. Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. 2020;38:1164–1174. doi: 10.1200/JCO.19.02745. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

