Validation of a Gene Expression Approach for the Cytological Diagnosis of Epithelioid and Biphasic Pleural Mesothelioma on a Consecutive Series
- PMID: 38067238
- PMCID: PMC10705177
- DOI: 10.3390/cancers15235534
Validation of a Gene Expression Approach for the Cytological Diagnosis of Epithelioid and Biphasic Pleural Mesothelioma on a Consecutive Series
Abstract
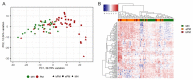
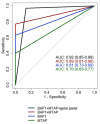
Cytological diagnosis of pleural mesothelioma (PM) is controversial, even using ancillary markers (BAP1, MTAP and CDKN2A). Here, we aimed to prospectively validate a previously developed 117-gene expression panel for the differential cytological diagnosis of epithelioid, biphasic PM and mesothelial hyperplasia. Seventy-seven pleural effusions were classified using the 117-gene expression levels (NanoString system). Sixty-eight cases were also screened for ancillary markers. The performance of both gene panel and ancillary markers was evaluated using ROC metrics. A score using the top consistently deregulated genes between epithelioid and biphasic PM was built to subtype malignant effusions. The panel alone reached a diagnostic accuracy (0.89) comparable to the best marker combination (BAP1 plus MTAP: 0.88). Ancillary tests missed 8 PMs, 7 of which were correctly classified by the panel. The score built by averaging the expression levels of MSLN, CLDN15 and CFB showed an accuracy of 0.80 in subtyping epithelioid and biphasic effusions. The 117-gene panel is effective for PM cytological diagnosis of epithelioid and biphasic PM. This tool can be complementary to ancillary markers, reducing invasive procedures and allowing an earlier diagnosis. Finally, the possibility to subtype PM on effusions strengthens the panel's role in PM diagnosis and management.
Keywords: gene expression; mesothelial hyperplasia; pleural effusions; pleural mesothelioma; subtyping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Gene Expression Analysis of Biphasic Pleural Mesothelioma: New Potential Diagnostic and Prognostic Markers.Diagnostics (Basel). 2022 Mar 10;12(3):674. doi: 10.3390/diagnostics12030674. Diagnostics (Basel). 2022. PMID: 35328227 Free PMC article.
-
Diagnostic capacity of BAP1 and MTAP in cytology from effusions and biopsy in mesothelioma.J Am Soc Cytopathol. 2022 Nov-Dec;11(6):385-393. doi: 10.1016/j.jasc.2022.07.003. Epub 2022 Jul 9. J Am Soc Cytopathol. 2022. PMID: 35945149
-
Differential Diagnosis of Malignant Pleural Mesothelioma on Cytology: A Gene Expression Panel versus BRCA1-Associated Protein 1 and p16 Tests.J Mol Diagn. 2020 Apr;22(4):457-466. doi: 10.1016/j.jmoldx.2019.12.009. Epub 2020 Feb 7. J Mol Diagn. 2020. PMID: 32036091
-
The cytological features of effusions with mesothelioma in situ: A report of 9 cases.Diagn Cytopathol. 2023 Jun;51(6):374-388. doi: 10.1002/dc.25129. Epub 2023 Mar 21. Diagn Cytopathol. 2023. PMID: 36942732 Review.
-
Update of pathological diagnosis of pleural mesothelioma using genomic-based morphological techniques, for both histological and cytological investigations.Pathol Int. 2022 Aug;72(8):389-401. doi: 10.1111/pin.13235. Epub 2022 May 21. Pathol Int. 2022. PMID: 35596704 Review.
References
-
- Sauter J.L., Dacic S., Galateau-Salle F., Attanoos R.L., Butnor K.J., Churg A., Husain A.N., Kadota K., Khoor A., Nicholson A.G., et al. The 2021 WHO Classification of Tumors of the Pleura: Advances Since the 2015 Classification. J. Thorac. Oncol. 2022;17:608–622. doi: 10.1016/j.jtho.2021.12.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

