A Single Dose of PEG-Asparaginase at the Beginning of Induction Not Only Accelerates MRD Clearance but Also Improves Long-Term Outcome in Children with B-Lineage ALL
- PMID: 38067249
- PMCID: PMC10705323
- DOI: 10.3390/cancers15235547
A Single Dose of PEG-Asparaginase at the Beginning of Induction Not Only Accelerates MRD Clearance but Also Improves Long-Term Outcome in Children with B-Lineage ALL
Abstract
This report presents the results of the assessment of MRD response by multicolor flow cytometry (MFC) with regard to the randomized use of pegylated asparaginase (PEG). In this study, PEG was randomly administered at a dose of 1000 U/m2 on day 3 of induction therapy in children with B-lineage ALL.
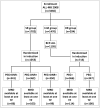
Methods: Conventional induction therapy consisted of dexamethasone, vincristine, and daunorubicin. MRD data was available in 502 patients who were randomized at the start of induction therapy, standard-risk (SR) patients into three (conventional induction without PEG, induction with additional PEG and with PEG but without daunorubicin) and intermediate-risk (ImR) patients into two groups (with additional PEG and without PEG).
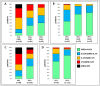
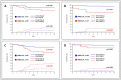
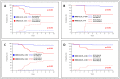
Results: The single administration of PEG resulted in a significantly higher proportion of rapid responders, in SR patients even when no anthracyclines were used for induction. In the SR group, the event-free survival of the MFC-MRD fast responders was similar in the PEG- and PEG+ arms (92.0 ± 3.1% vs. 96.2 ± 1.5%, respectively), and the same unfavorable trend was observed for MFC-MRD slow responders (57.5 ± 12.3% vs. 66.7 ± 15.7%, respectively). Results were similar in ImR patients: (94.3 ± 3.2% vs. 95.1 ± 2.4%, for fast responders and 63.3 ± 7.6% vs. 78.1 ± 7.9%, for slow responders in PEG- and PEG+ arms, respectively). However, there is a large difference between the proportion of MFC-MRD slow responders in the PEG- and PEG+ groups (18.3% vs. 5.2% for the SR group and 44.2% vs. 25.0% for the ImR group).
Conclusions: Therefore, early use of PEG-ASP not only leads to an accelerated reduction of blasts, but also to an excellent outcome in a significantly larger proportion of patients in both risk groups.
Keywords: PEG-asparaginase; acute lymphoblastic leukemia; flow cytometry; minimal residual disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A simple procedure to identify children with B-lineage acute lymphoblastic leukemia who can be successfully treated with low or moderate intensity: Sequential versus single-point minimal residual disease measurement.Pediatr Blood Cancer. 2023 Jun;70(6):e30295. doi: 10.1002/pbc.30295. Epub 2023 Mar 28. Pediatr Blood Cancer. 2023. PMID: 36975157
-
Methodological aspects of minimal residual disease assessment by flow cytometry in acute lymphoblastic leukemia: A French multicenter study.Cytometry B Clin Cytom. 2015 Jan;88(1):21-9. doi: 10.1002/cyto.b.21195. Epub 2014 Nov 1. Cytometry B Clin Cytom. 2015. PMID: 25363877 Clinical Trial.
-
[Minimal residual disease analysis in acute lymphoblastic leukemia of childhood within the framework of COALL Study: results of an induction therapy without asparaginase].Klin Padiatr. 2000 Jul-Aug;212(4):169-73. doi: 10.1055/s-2000-9672. Klin Padiatr. 2000. PMID: 10994545 Clinical Trial. German.
-
Flow Cytometric Minimal Residual Disease Analysis in Acute Leukemia: Current Status.Indian J Hematol Blood Transfus. 2020 Jan;36(1):3-15. doi: 10.1007/s12288-019-01118-5. Epub 2019 Apr 2. Indian J Hematol Blood Transfus. 2020. PMID: 32174688 Free PMC article. Review.
-
Measurement of minimal residual disease before and after myeloablative hematopoietic cell transplantation for acute leukemia.Best Pract Res Clin Haematol. 2013 Sep;26(3):279-84. doi: 10.1016/j.beha.2013.10.008. Epub 2013 Oct 16. Best Pract Res Clin Haematol. 2013. PMID: 24309531 Review.
Cited by
-
Exploration of the intracellular chiral metabolome in pediatric BCP-ALL: a pilot study investigating the metabolic phenotype of IgH locus aberrations.Front Oncol. 2024 Aug 5;14:1413264. doi: 10.3389/fonc.2024.1413264. eCollection 2024. Front Oncol. 2024. PMID: 39161381 Free PMC article.
-
Efficacy and Safety Profile of Biosimilar Polyethylene Glycol (PEG)-Asparaginase (Asviia) in Patients With Acute Leukemia: A Retrospective Study From Kashmir.Cureus. 2024 Nov 15;16(11):e73727. doi: 10.7759/cureus.73727. eCollection 2024 Nov. Cureus. 2024. PMID: 39677089 Free PMC article.
References
-
- Rizzari C., Citterio M., Zucchetti M., Conter V., Chiesa R., Colombini A., Malguzzi S., Silvestri D., D’Incalci M. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica. 2006;91:24–31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

