Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal-Epithelial Transition in Hepatocellular Carcinoma
- PMID: 38067256
- PMCID: PMC10705132
- DOI: 10.3390/cancers15235553
Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal-Epithelial Transition in Hepatocellular Carcinoma
Abstract
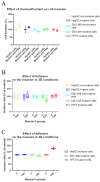
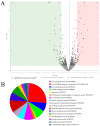
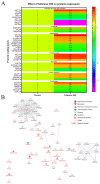
The epithelial-mesenchymal transition (EMT) is a process in which epithelial cells acquire the ability to actively migrate via a change to the mesenchymal phenotype. This mechanism occurs in an environment rich in cytokines and reactive oxygen species but poor in nutrients. The aim of this study was to demonstrate that the use of a fullerene C60 nanofilm can inhibit liver cancer cell invasion by restoring their non-aggressive, epithelial phenotype. We employed epithelial and mesenchymal HepG2 and SNU-449 liver cancer cells and non-cancerous mesenchymal HFF2 cells in this work. We used enzyme-linked immunosorbent assays (ELISAs) to determine the content of glutathione and transforming growth factor (TGF) in cells. We measured the total antioxidant capacity with a commercially available kit. We assessed cell invasion based on changes in morphology, the scratch test and the Boyden chamber invasion. In addition, we measured the effect of C60 nanofilm on restoring the epithelial phenotype at the protein level with protein membranes, Western blotting and mass spectrometry. C60 nanofilm downregulated TGF and increased glutathione expression in SNU-449 cells. When grown on C60 nanofilm, invasive cells showed enhanced intercellular connectivity; reduced three-dimensional invasion; and reduced the expression of key invasion markers, namely MMP-1, MMP-9, TIMP-1, TIMP-2 and TIMP-4. Mass spectrometry showed that among the 96 altered proteins in HepG2 cells grown on C60 nanofilm, 41 proteins are involved in EMT and EMT-modulating processes such as autophagy, inflammation and oxidative stress. The C60 nanofilm inhibited autophagy, showed antioxidant and anti-inflammatory properties, increased glucose transport and regulated the β-catenin/keratin/Smad4/snail+slug and MMP signalling pathways. In conclusion, the C60 nanofilm induces a hybrid mesenchymal-epithelial phenotype and could be used in the prevention of postoperative recurrences.
Keywords: autophagy; fullerene; inflammation; invasion; liver cancer; oxidative stress; phenotype transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Polyhydroxylated Fullerene C60(OH)40 Nanofilms Promote the Mesenchymal-Epithelial Transition of Human Liver Cancer Cells via the TGF-β1/Smad Pathway.J Inflamm Res. 2023 Aug 29;16:3739-3761. doi: 10.2147/JIR.S415378. eCollection 2023. J Inflamm Res. 2023. PMID: 37663761 Free PMC article.
-
Autophagy induces transforming growth factor-β-dependent epithelial-mesenchymal transition in hepatocarcinoma cells through cAMP response element binding signalling.J Cell Mol Med. 2018 Nov;22(11):5518-5532. doi: 10.1111/jcmm.13825. Epub 2018 Aug 22. J Cell Mol Med. 2018. PMID: 30134011 Free PMC article.
-
Mechano-signalling, induced by fullerene C60 nanofilms, arrests the cell cycle in the G2/M phase and decreases proliferation of liver cancer cells.Int J Nanomedicine. 2019 Aug 6;14:6197-6215. doi: 10.2147/IJN.S206934. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31496681 Free PMC article.
-
Caffeic Acid and Metformin Inhibit Invasive Phenotype Induced by TGF-β1 in C-4I and HTB-35/SiHa Human Cervical Squamous Carcinoma Cells by Acting on Different Molecular Targets.Int J Mol Sci. 2018 Jan 16;19(1):266. doi: 10.3390/ijms19010266. Int J Mol Sci. 2018. PMID: 29337896 Free PMC article.
-
Fangchinoline targets epithelial-mesenchymal transition process by modulating activation of multiple cell-signaling pathways.J Cell Biochem. 2022 Jul;123(7):1222-1236. doi: 10.1002/jcb.30279. Epub 2022 May 27. J Cell Biochem. 2022. PMID: 35621239
References
-
- Shinde A., Hardy S.D., Kim D., Akhand S.S., Jolly M.K., Wang W.H., Anderson J.C., Khodadadi R.B., Brown W.S., George J.T., et al. Spleen tyrosine kinase–mediated autophagy is required for epithelial–mesenchymal plasticity and metastasis in breast cancer. Cancer Res. 2019;79:1831–1843. doi: 10.1158/0008-5472.CAN-18-2636. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

