Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial
- PMID: 38067283
- PMCID: PMC10705113
- DOI: 10.3390/cancers15235579
Five-Fraction Proton Therapy for the Treatment of Skull Base Chordomas and Chondrosarcomas: Early Results of a Prospective Series and Description of a Clinical Trial
Abstract
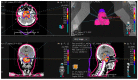
(1) Background: Our purpose is to describe the design of a phase II clinical trial on 5-fraction proton therapy for chordomas and chondrosarcomas of the skull base and to present early results in terms of local control and clinical tolerance of the first prospective series. (2) Methods: A dose of 37.5 GyRBE in five fractions was proposed for chordomas and 35 GyRBE in five fractions for chondrosarcomas. The established inclusion criteria are age ≥ 18 years, Karnofsky Performance Status ≥ 70%, clinical target volume up to 50 cc, and compliance with dose restrictions to the critical organs. Pencil beam scanning was used for treatment planning, employing four to six beams. (3) Results: A total of 11 patients (6 chordomas and 5 chondrosarcomas) were included. The median follow-up was 12 months (9-15 months) with 100% local control. Acute grade I-II headache (64%), grade I asthenia and alopecia (45%), grade I nausea (27%), and grade I dysphagia (18%) were described. Late toxicity was present in two patients with grade 3 temporal lobe necrosis. (4) Conclusions: Hypofractionated proton therapy is showing encouraging preliminary results. However, to fully assess the efficacy of this therapeutic approach, future trials with adequate sample sizes and extended follow-ups are necessary.
Keywords: chondrosarcoma; chordoma; hypofractionated; proton therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Proton therapy with a fixed beamline for skull-base chordomas and chondrosarcomas: outcomes and toxicity.Radiat Oncol. 2021 Dec 20;16(1):238. doi: 10.1186/s13014-021-01961-9. Radiat Oncol. 2021. PMID: 34930352 Free PMC article.
-
Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report.Int J Radiat Oncol Biol Phys. 2009 Nov 15;75(4):1111-8. doi: 10.1016/j.ijrobp.2008.12.055. Epub 2009 Apr 20. Int J Radiat Oncol Biol Phys. 2009. PMID: 19386442
-
Proton Therapy for Skull-Base Chordomas and Chondrosarcomas: Initial Results From the Beaumont Proton Therapy Center.Cureus. 2021 May 27;13(5):e15278. doi: 10.7759/cureus.15278. Cureus. 2021. PMID: 34194880 Free PMC article.
-
Proton radiation therapy for chordomas and chondrosarcomas of the skull base.Neurosurg Clin N Am. 2000 Oct;11(4):627-38. Neurosurg Clin N Am. 2000. PMID: 11082173 Review.
-
Pure proton therapy for skull base chordomas and chondrosarcomas: A systematic review of clinical experience.Front Oncol. 2022 Nov 25;12:1016857. doi: 10.3389/fonc.2022.1016857. eCollection 2022. Front Oncol. 2022. PMID: 36505855 Free PMC article.
Cited by
-
Post-treatment late and long-term effects in bone sarcoma: A scoping review.J Bone Oncol. 2025 Mar 21;52:100671. doi: 10.1016/j.jbo.2025.100671. eCollection 2025 Jun. J Bone Oncol. 2025. PMID: 40206491 Free PMC article. Review.
-
Skull-Base Chondrosarcoma: A Systematic Review of the Role of Postoperative Radiotherapy.Cancers (Basel). 2024 Feb 21;16(5):856. doi: 10.3390/cancers16050856. Cancers (Basel). 2024. PMID: 38473218 Free PMC article. Review.
References
-
- Bakker S.H., Jacobs W.C.H., Pondaag W., Gelderblom H., Nout R.A., Dijkstra P.D.S., Peul W.C., Vleggeert-Lankamp C.L.A. Chordoma: A systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur. Spine J. 2018;27:3043–3058. doi: 10.1007/s00586-018-5764-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources