Epigenetically Downregulated Breast Cancer Gene 2 through Acetyltransferase Lysine Acetyltransferase 2B Increases the Sensitivity of Colorectal Cancer to Olaparib
- PMID: 38067284
- PMCID: PMC10705808
- DOI: 10.3390/cancers15235580
Epigenetically Downregulated Breast Cancer Gene 2 through Acetyltransferase Lysine Acetyltransferase 2B Increases the Sensitivity of Colorectal Cancer to Olaparib
Abstract
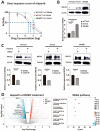
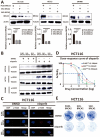
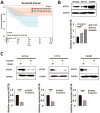
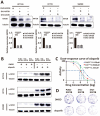
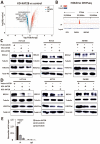
Olaparib suppresses DNA damage repair by inhibiting the poly ADP ribose polymerase (PARP), especially in cancers with BRCA1/2 mutations or the BRCA-ness phenotype. However, the first trials showed that some patients with defective DNA damage repair are still resistant to olaparib. The recovery of the wildtype BRCA is a prominent mechanism of PARP inhibitor (PARPi) resistance in BRCA-deficient tumors, but additional molecular features of olaparib resistance remain poorly understood. The objective of our study was to find molecular parameters that contribute to olaparib response or resistance in CRC. We report that histone acetyltransferase KAT2B decreases BRCA2 expression by reducing the acetylation of the 27th amino acid in histone H3 (H3K27) at the promoter of the BRCA2 gene in colorectal cancer (CRC). This increases the sensitivity of CRC cells toward olaparib treatment. The H3K27ac binding domain of BRCA2 may be required for its transcription. Low endogenous KAT2B expression, which we identify in a subset of cultured BRCA2-expressing CRC cells, leads to an accumulation of γH2AX (more DNA damage), resulting in low PARPi resistance in BRCA-expressing cells. Our results reveal KAT2B and histone acetylation as regulators of BRCA2 expression and PARPi responses in BRCA2-expressing CRC cells, providing further insights into molecular prerequisites for targeting BRCA-functional tumors.
Keywords: BRCA2; H3K27ac; KAT2B; colorectal cancer; olaparib sensitivity; γH2AX.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
PCAF-Mediated Histone Acetylation Promotes Replication Fork Degradation by MRE11 and EXO1 in BRCA-Deficient Cells.Mol Cell. 2020 Oct 15;80(2):327-344.e8. doi: 10.1016/j.molcel.2020.08.018. Epub 2020 Sep 22. Mol Cell. 2020. PMID: 32966758 Free PMC article.
-
Combining Carbon-Ion Irradiation and PARP Inhibitor, Olaparib Efficiently Kills BRCA1-Mutated Triple-Negative Breast Cancer Cells.Breast Cancer (Auckl). 2022 Mar 23;16:11782234221080553. doi: 10.1177/11782234221080553. eCollection 2022. Breast Cancer (Auckl). 2022. PMID: 35340889 Free PMC article.
-
PARP inhibitor resistance and TP53 mutations in patients treated with olaparib for BRCA-mutated cancer: Four case reports.Mol Med Rep. 2021 Jan;23(1):75. doi: 10.3892/mmr.2020.11713. Epub 2020 Nov 25. Mol Med Rep. 2021. PMID: 33236159
-
Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: the rationale for their inclusion in the clinic.J Exp Clin Cancer Res. 2016 Nov 24;35(1):179. doi: 10.1186/s13046-016-0456-2. J Exp Clin Cancer Res. 2016. PMID: 27884198 Free PMC article. Review.
-
Role of BRCA Mutations in Cancer Treatment with Poly(ADP-ribose) Polymerase (PARP) Inhibitors.Cancers (Basel). 2018 Dec 4;10(12):487. doi: 10.3390/cancers10120487. Cancers (Basel). 2018. PMID: 30518089 Free PMC article. Review.
Cited by
-
Celastrol promotes DNA damage and apoptosis in uterine corpus endometrial carcinoma via promotion of KAT2B-mediated RBPJ acetylation and repression of MCM4 transcription.Mol Med. 2025 Feb 3;31(1):39. doi: 10.1186/s10020-025-01082-z. Mol Med. 2025. PMID: 39901144 Free PMC article.
References
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Phelan C.M., Iqbal J., Lynch H.T., Lubinski J., Gronwald J., Moller P., Ghadirian P., Foulkes W.D., Armel S., Eisen A., et al. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: Results from a follow-up study. Br. J. Cancer. 2014;110:530–534. doi: 10.1038/bjc.2013.741. - DOI - PMC - PubMed
-
- Ishaque N., Abba M.L., Hauser C., Patil N., Paramasivam N., Huebschmann D., Leupold J.H., Balasubramanian G.P., Kleinheinz K., Toprak U.H., et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat. Commun. 2018;9:4782. doi: 10.1038/s41467-018-07041-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

