Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review
- PMID: 38067304
- PMCID: PMC10705512
- DOI: 10.3390/cancers15235600
Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review
Abstract
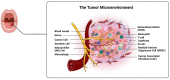
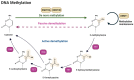
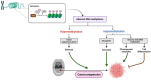
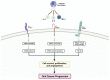
Oral squamous cell carcinoma (OSCC) is a prevalent and significant type of oral cancer that has far-reaching health implications worldwide. Epigenetics, a field focused on studying heritable changes in gene expression without modifying DNA sequence, plays a pivotal role in OSCC. Epigenetic changes, encompassing DNA methylation, histone modifications, and miRNAs, exert control over gene activity and cellular characteristics. In OSCC, aberrant DNA methylation of tumor suppressor genes (TSG) leads to their inactivation, subsequently facilitating tumor growth. As a result, distinct patterns of gene methylation hold promise as valuable biomarkers for the detection of OSCC. Oral cancer treatment typically involves surgery, radiation therapy, and chemotherapy, but even with these treatments, cancer cells cannot be effectively targeted and destroyed. Researchers are therefore exploring new methods to target and eliminate cancer cells. One promising approach is the use of epigenetic modifiers, such as DNA methyltransferase (DNMT) inhibitors and histone deacetylase (HDAC) inhibitors, which have been shown to modify abnormal epigenetic patterns in OSCC cells, leading to the reactivation of TSGs and the suppression of oncogenes. As a result, epigenetic-targeted therapies have the potential to directly alter gene expression and minimize side effects. Several studies have explored the efficacy of such therapies in the treatment of OSCC. Although studies have investigated the efficacy of epigenetic therapies, challenges in identifying reliable biomarkers and developing effective combination treatments are acknowledged. Of note, epigenetic mechanisms play a significant role in drug resistance in OSCC and other cancers. Aberrant DNA methylation can silence tumor suppressor genes, while alterations in histone modifications and chromatin remodeling affect gene expression related to drug metabolism and cell survival. Thus, understanding and targeting these epigenetic processes offer potential strategies to overcome drug resistance and improve the efficacy of cancer treatments in OSCC. This comprehensive review focuses on the complex interplay between epigenetic alterations and OSCC cells. This will involve a deep dive into the mechanisms underlying epigenetic modifications and their impact on OSCC, including its initiation, progression, and metastasis. Furthermore, this review will present the role of epigenetics in the treatment and diagnosis of OSCC.
Keywords: DNA methylation; drug resistance; epigenetic; oral squamous cell carcinoma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
DNA hypermethylation of tumor suppressor genes among oral squamous cell carcinoma patients: a prominent diagnostic biomarker.Mol Biol Rep. 2024 Dec 7;52(1):44. doi: 10.1007/s11033-024-10144-0. Mol Biol Rep. 2024. PMID: 39644423 Review.
-
Promoter Hypermethylation of LATS2 Gene in Oral Squamous Cell Carcinoma (OSCC) Among North Indian Population.Asian Pac J Cancer Prev. 2020 May 1;21(5):1283-1287. doi: 10.31557/APJCP.2020.21.5.1283. Asian Pac J Cancer Prev. 2020. PMID: 32458634 Free PMC article.
-
Aberrantly hypermethylated tumor suppressor genes were identified in oral squamous cell carcinoma (OSCC).Clin Epigenetics. 2019 Aug 12;11(1):116. doi: 10.1186/s13148-019-0715-0. Clin Epigenetics. 2019. PMID: 31405379 Free PMC article.
-
Impact of Epigenetic Alterations in the Development of Oral Diseases.Curr Med Chem. 2021;28(6):1091-1103. doi: 10.2174/0929867327666200114114802. Curr Med Chem. 2021. PMID: 31942842 Review.
-
Epigenetics in oral squamous cell carcinoma.J Oral Maxillofac Pathol. 2017 May-Aug;21(2):252-259. doi: 10.4103/jomfp.JOMFP_150_17. J Oral Maxillofac Pathol. 2017. PMID: 28932035 Free PMC article. Review.
Cited by
-
Immunohistochemical Expression of DAPK-1 in Oral Leukoplakia And Oral Squamous Cell Carcinoma: A Preliminary Study.Cureus. 2025 Feb 16;17(2):e79085. doi: 10.7759/cureus.79085. eCollection 2025 Feb. Cureus. 2025. PMID: 40104466 Free PMC article.
-
DNA hypermethylation of tumor suppressor genes among oral squamous cell carcinoma patients: a prominent diagnostic biomarker.Mol Biol Rep. 2024 Dec 7;52(1):44. doi: 10.1007/s11033-024-10144-0. Mol Biol Rep. 2024. PMID: 39644423 Review.
-
Porphyromonas gingivalis outer membrane vesicles augments proliferation and metastasis of oral squamous cell carcinoma cells.BMC Oral Health. 2025 May 10;25(1):701. doi: 10.1186/s12903-025-05937-z. BMC Oral Health. 2025. PMID: 40348995 Free PMC article.
-
Dysregulated PI3K/AKT signaling in oral squamous cell carcinoma: The tumor microenvironment and epigenetic modifiers as key drivers.Oncol Res. 2025 Jul 18;33(8):1835-1860. doi: 10.32604/or.2025.064010. eCollection 2025. Oncol Res. 2025. PMID: 40746882 Free PMC article. Review.
-
Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment.Biology (Basel). 2025 Jul 10;14(7):842. doi: 10.3390/biology14070842. Biology (Basel). 2025. PMID: 40723400 Free PMC article. Review.
References
-
- Nicolette Salmon H., Jan-MichaÉL H., Jamshid J., Miranda M.J., Lars S. Viral and Molecular Aspects of Oral Cancer. Anticancer Res. 2012;32:4201. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

