Cellular Plasticity in Mammary Gland Development and Breast Cancer
- PMID: 38067308
- PMCID: PMC10705338
- DOI: 10.3390/cancers15235605
Cellular Plasticity in Mammary Gland Development and Breast Cancer
Abstract
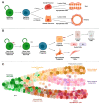
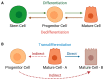
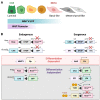
Cellular plasticity is a phenomenon where cells adopt different identities during development and tissue homeostasis as a response to physiological and pathological conditions. This review provides a general introduction to processes by which cells change their identity as well as the current definition of cellular plasticity in the field of mammary gland biology. Following a synopsis of the evolving model of the hierarchical development of mammary epithelial cell lineages, we discuss changes in cell identity during normal mammary gland development with particular emphasis on the effect of the gestation cycle on the emergence of new cellular states. Next, we summarize known mechanisms that promote the plasticity of epithelial lineages in the normal mammary gland and highlight the importance of the microenvironment and extracellular matrix. A discourse of cellular reprogramming during the early stages of mammary tumorigenesis that follows focuses on the origin of basal-like breast cancers from luminal progenitors and oncogenic signaling networks that orchestrate diverse developmental trajectories of transforming epithelial cells. In addition to the epithelial-to-mesenchymal transition, we highlight events of cellular reprogramming during breast cancer progression in the context of intrinsic molecular subtype switching and the genesis of the claudin-low breast cancer subtype, which represents the far end of the spectrum of epithelial cell plasticity. In the final section, we will discuss recent advances in the design of genetically engineered models to gain insight into the dynamic processes that promote cellular plasticity during mammary gland development and tumorigenesis in vivo.
Keywords: breast cancer; cellular plasticity; epithelial-to-mesenchymal transition; mammary gland development; mouse models; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):149-68. doi: 10.1007/s10911-010-9180-2. Epub 2010 May 12. J Mammary Gland Biol Neoplasia. 2010. PMID: 20461450 Review.
-
Hybrid Stem Cell States: Insights Into the Relationship Between Mammary Development and Breast Cancer Using Single-Cell Transcriptomics.Front Cell Dev Biol. 2020 May 8;8:288. doi: 10.3389/fcell.2020.00288. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32457901 Free PMC article.
-
Plasticity and Potency of Mammary Stem Cell Subsets During Mammary Gland Development.Int J Mol Sci. 2019 May 13;20(9):2357. doi: 10.3390/ijms20092357. Int J Mol Sci. 2019. PMID: 31085991 Free PMC article. Review.
-
Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):117-34. doi: 10.1007/s10911-010-9178-9. Epub 2010 May 19. J Mammary Gland Biol Neoplasia. 2010. PMID: 20490631 Free PMC article. Review.
-
Cellular Plasticity of Mammary Epithelial Cells Underlies Heterogeneity of Breast Cancer.Biomedicines. 2018 Nov 1;6(4):103. doi: 10.3390/biomedicines6040103. Biomedicines. 2018. PMID: 30388868 Free PMC article. Review.
Cited by
-
Molecular Docking and Pharmacokinetic Profiling of Nab-paclitaxel as Advanced Chemotherapeutic Agent Against HER-2 Positive Breast Cancer Patients.Asian Pac J Cancer Prev. 2024 Oct 1;25(10):3447-3456. doi: 10.31557/APJCP.2024.25.10.3447. Asian Pac J Cancer Prev. 2024. PMID: 39471010 Free PMC article.
-
The molecular features of lung cancer stem cells in dedifferentiation process-driven epigenetic alterations.J Biol Chem. 2024 Dec;300(12):107994. doi: 10.1016/j.jbc.2024.107994. Epub 2024 Nov 14. J Biol Chem. 2024. PMID: 39547513 Free PMC article. Review.
-
Intraductal Injection of Adenoviruses to Perform Lineage Tracing in the Mammary Gland.J Mammary Gland Biol Neoplasia. 2025 Jul 7;30(1):10. doi: 10.1007/s10911-025-09584-6. J Mammary Gland Biol Neoplasia. 2025. PMID: 40622584 Free PMC article. Review.
-
Overexpression of TSG101 causes the development of adenosquamous mammary carcinoma.Breast Cancer Res. 2025 Jul 7;27(1):126. doi: 10.1186/s13058-025-02007-8. Breast Cancer Res. 2025. PMID: 40624726 Free PMC article.
-
LMO2 regulates epithelial-mesenchymal plasticity of mammary epithelial cells.bioRxiv [Preprint]. 2025 May 27:2025.05.22.655436. doi: 10.1101/2025.05.22.655436. bioRxiv. 2025. PMID: 40501810 Free PMC article. Preprint.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

