Spatial Distribution of Non-Immune Cells Expressing Glycoprotein A Repetitions Predominant in Human and Murine Metastatic Lymph Nodes
- PMID: 38067325
- PMCID: PMC10705676
- DOI: 10.3390/cancers15235621
Spatial Distribution of Non-Immune Cells Expressing Glycoprotein A Repetitions Predominant in Human and Murine Metastatic Lymph Nodes
Abstract
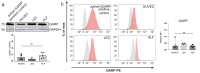
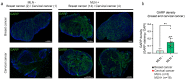
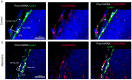
Several types of cancer spread through the lymphatic system via the sentinel lymph nodes (LNs). Such LN-draining primary tumors, modified by tumor factors, lead to the formation of a metastatic niche associated with an increased number of Foxp3+ regulatory T cells (Tregs). These cells are expected to contribute to the elaboration of an immune-suppressive environment. Activated Tregs express glycoprotein A repetitions predominant (GARP), which binds and presents latent transforming growth factor beta 1 (TGF-β1) at their surface. GARP is also expressed by other non-immune cell types poorly described in LNs. Here, we mapped GARP expression in non-immune cells in human and mouse metastatic LNs. The mining of available (human and murine) scRNA-Seq datasets revealed GARP expression by blood (BEC)/lymphatic (LEC) endothelial, fibroblastic, and perivascular cells. Consistently, through immunostaining and in situ RNA hybridization approaches, GARP was detected in and around blood and lymphatic vessels, in (αSMA+) fibroblasts, and in perivascular cells associated with an abundant matrix. Strikingly, GARP was detected in LECs forming the subcapsular sinus and high endothelial venules (HEVs), two vascular structures localized at the interface between LNs and the afferent lymphatic and blood vessels. Altogether, we here provide the first distribution maps for GARP in human and murine LNs.
Keywords: GARP mRNA; LRRC32; cancer; glycoprotein A repetitions predominant (GARP); lymph node; metastases; transforming growth factor beta 1 (TGF-β1); tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Lysosomal-associated Transmembrane Protein 4B (LAPTM4B) Decreases Transforming Growth Factor β1 (TGF-β1) Production in Human Regulatory T Cells.J Biol Chem. 2015 Aug 14;290(33):20105-16. doi: 10.1074/jbc.M115.655340. Epub 2015 Jun 30. J Biol Chem. 2015. PMID: 26126825 Free PMC article.
-
GARP-TGF-β complexes negatively regulate regulatory T cell development and maintenance of peripheral CD4+ T cells in vivo.J Immunol. 2013 May 15;190(10):5057-64. doi: 10.4049/jimmunol.1300065. Epub 2013 Apr 10. J Immunol. 2013. PMID: 23576681 Free PMC article.
-
Expression of GARP Is Increased in Tumor-Infiltrating Regulatory T Cells and Is Correlated to Clinicopathology of Lung Cancer Patients.Front Immunol. 2017 Feb 14;8:138. doi: 10.3389/fimmu.2017.00138. eCollection 2017. Front Immunol. 2017. PMID: 28261204 Free PMC article.
-
Garp as a therapeutic target for modulation of T regulatory cell function.Expert Opin Ther Targets. 2017 Feb;21(2):191-200. doi: 10.1080/14728222.2017.1275568. Epub 2016 Dec 29. Expert Opin Ther Targets. 2017. PMID: 28001437 Review.
-
GARP as a Therapeutic Target for the Modulation of Regulatory T Cells in Cancer and Autoimmunity.Front Immunol. 2022 Jul 8;13:928450. doi: 10.3389/fimmu.2022.928450. eCollection 2022. Front Immunol. 2022. PMID: 35898500 Free PMC article. Review.
References
-
- Balsat C., Blacher S., Herfs M., Van de Velde M., Signolle N., Sauthier P., Pottier C., Gofflot S., De Cuypere M., Delvenne P., et al. A Specific Immune and Lymphatic Profile Characterizes the Pre-Metastatic State of the Sentinel Lymph Node in Patients with Early Cervical Cancer. Oncoimmunology. 2017;6:e1265718. doi: 10.1080/2162402X.2016.1265718. - DOI - PMC - PubMed
-
- Wakisaka N., Hasegawa Y., Yoshimoto S., Miura K., Shiotani A., Yokoyama J., Sugasawa M., Moriyama-Kita M., Endo K., Yoshizaki T. Primary Tumor-Secreted Lymphangiogenic Factors Induce Pre-Metastatic Lymphvascular Niche Formation at Sentinel Lymph Nodes in Oral Squamous Cell Carcinoma. PLoS ONE. 2015;10:e0144056. doi: 10.1371/journal.pone.0144056. - DOI - PMC - PubMed
-
- Maus R.L.G., Jakub J.W., Hieken T.J., Nevala W.K., Christensen T.A., Sutor S.L., Flotte T.J., Markovic S.N. Identification of Novel, Immune-Mediating Extracellular Vesicles in Human Lymphatic Effluent Draining Primary Cutaneous Melanoma. OncoImmunology. 2019;8:e1667742. doi: 10.1080/2162402X.2019.1667742. - DOI - PMC - PubMed
Grants and funding
- PDR-Télévie, PDR/Fund for Scientific Research
- grants 2020-107 for FK, 2022-181 for AN ; 2020-079 for SL/Stichting tegen Kanker
- EOS No. 0.0037.22/FNRS-FWO
- Fonds spéciaux de la Recherche, University of Liege
- Fondation Hospitalo Universitaire Léon Fredericq (FHULF, University of Liège), the Fondation Salus Sanguinis
LinkOut - more resources
Full Text Sources

