Targeting Proteasomes and the MHC Class I Antigen Presentation Machinery to Treat Cancer, Infections and Age-Related Diseases
- PMID: 38067336
- PMCID: PMC10705104
- DOI: 10.3390/cancers15235632
Targeting Proteasomes and the MHC Class I Antigen Presentation Machinery to Treat Cancer, Infections and Age-Related Diseases
Abstract
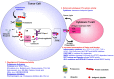
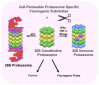
The majority of T-cell responses involve proteasome-dependent protein degradation and the downstream presentation of oligopeptide products complexed with major histocompatibility complex (MHC) class I (MHC-I) molecules to peptide-restricted CD8+ T-cells. However, evasion of host immunity is a cancer hallmark that is achieved by disruption of host antigen processing and presentation machinery (APM). Consequently, mechanisms of immune evasion promote cancer growth and survival as well as de novo and acquired resistance to immunotherapy. A multitude of cell signaling pathways modulate the APM and MHC-I-dependent antigen presentation. Pharmacologics that specifically target and modulate proteasome structure and activity represent a novel emerging strategy to improve the treatment of cancers and other diseases characterized by aberrant protein accumulation. FDA-approved pharmacologics that selectively activate proteasomes and/or immunoproteasomes can be repositioned to overcome the current bottlenecks that hinder drug development to enhance antigen presentation, modulate the immunopeptidome, and enhance the cytotoxic activity of endogenous or engineered T-cells. Strategies to enhance antigen presentation may also improve the antitumor activity of T-cell immunotherapies, checkpoint inhibitors, and cancer vaccines. Proteasomes represent actionable therapeutic targets to treat difficult-to-treat infectious processes and neurodegenerative diseases that are characterized by the unwanted accrual of insoluble, deleterious, and potentially toxic proteins. Taken together, we highlight the breadth and magnitude of the proteasome and the immense potential to amplify and unmask the immunopeptidomic landscape to improve the treatment of a spectrum of human diseases.
Keywords: antigen presentation; immune checkpoint inhibitors; immunopeptidome; immunoproteasome; proteasome inhibitors; ubiquitin–proteasome system.
Conflict of interest statement
The authors state that there are no conflict of interest.
Figures
Similar articles
-
HDAC6 Inhibition Releases HR23B to Activate Proteasomes, Expand the Tumor Immunopeptidome and Amplify T-cell Antimyeloma Activity.Cancer Res Commun. 2024 Jun 18;4(6):1517-1532. doi: 10.1158/2767-9764.CRC-23-0528. Cancer Res Commun. 2024. PMID: 38747592 Free PMC article.
-
The role of the ubiquitin-proteasome pathway in MHC class I antigen processing: implications for vaccine design.Curr Mol Med. 2001 Dec;1(6):665-76. doi: 10.2174/1566524013363230. Curr Mol Med. 2001. PMID: 11899255 Review.
-
Recent Advances in Lung Cancer Immunotherapy: Input of T-Cell Epitopes Associated With Impaired Peptide Processing.Front Immunol. 2019 Jul 3;10:1505. doi: 10.3389/fimmu.2019.01505. eCollection 2019. Front Immunol. 2019. PMID: 31333652 Free PMC article. Review.
-
Activated SUMOylation restricts MHC class I antigen presentation to confer immune evasion in cancer.J Clin Invest. 2022 May 2;132(9):e152383. doi: 10.1172/JCI152383. J Clin Invest. 2022. PMID: 35499080 Free PMC article.
-
Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation.Front Immunol. 2021 Mar 9;12:636568. doi: 10.3389/fimmu.2021.636568. eCollection 2021. Front Immunol. 2021. PMID: 33767702 Free PMC article. Review.
Cited by
-
Human Beta Defensin-2 mRNA and Proteasome Subunit β Type 8 mRNA Analysis, Useful in Differentiating Skin Biopsies from Atopic Dermatitis and Psoriasis Vulgaris Patients.Int J Mol Sci. 2024 Aug 24;25(17):9192. doi: 10.3390/ijms25179192. Int J Mol Sci. 2024. PMID: 39273140 Free PMC article.
-
Tetrandrine augments melanoma cell immunogenicity via dual inhibition of autophagic flux and proteasomal activity enhancing MHC-I presentation.Acta Pharmacol Sin. 2025 Jul;46(7):2056-2072. doi: 10.1038/s41401-025-01507-9. Epub 2025 Feb 27. Acta Pharmacol Sin. 2025. PMID: 40016522 Free PMC article.
-
Immunoproteasome Activation Expands the MHC Class I Immunopeptidome, Unmasks Neoantigens, and Enhances T-cell Anti-Myeloma Activity.Mol Cancer Ther. 2024 Dec 3;23(12):1743-1760. doi: 10.1158/1535-7163.MCT-23-0931. Mol Cancer Ther. 2024. PMID: 39210605 Free PMC article.
-
Genomics and proteomics to determine novel molecular subtypes and predict the response to immunotherapy and the effect of bevacizumab in glioblastoma.Sci Rep. 2024 Jul 31;14(1):17630. doi: 10.1038/s41598-024-68648-5. Sci Rep. 2024. PMID: 39085480 Free PMC article.
-
Defects in antigen processing and presentation: mechanisms, immune evasion and implications for cancer vaccine development.Nat Rev Immunol. 2025 Aug 8. doi: 10.1038/s41577-025-01208-8. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40781552 Review.
References
-
- Schoenheimer R. The Dynamic State of Body Constituents. Harvard University Press; Cambridge, MA, USA: 1942.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

