Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics
- PMID: 38067344
- PMCID: PMC10705070
- DOI: 10.3390/cancers15235639
Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics
Abstract
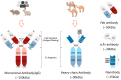
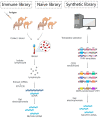
Monoclonal antibodies (mAbs) have exhibited substantial potential as targeted therapeutics in cancer treatment due to their precise antigen-binding specificity. Despite their success in tumor-targeted therapies, their effectiveness is hindered by their large size and limited tissue permeability. Camelid-derived single-domain antibodies, also known as nanobodies, represent the smallest naturally occurring antibody fragments. Nanobodies offer distinct advantages over traditional mAbs, including their smaller size, high stability, lower manufacturing costs, and deeper tissue penetration capabilities. They have demonstrated significant roles as both diagnostic and therapeutic tools in cancer research and are also considered as the next generation of antibody drugs. In this review, our objective is to provide readers with insights into the development and various applications of nanobodies in the field of cancer treatment, along with an exploration of the challenges and strategies for their prospective clinical trials.
Keywords: EGFR; HER2; NIR; PD-L1; VHH; immunoPET.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics.Front Oncol. 2020 Jul 23;10:1182. doi: 10.3389/fonc.2020.01182. eCollection 2020. Front Oncol. 2020. PMID: 32793488 Free PMC article. Review.
-
NANOBODIES®: A Review of Diagnostic and Therapeutic Applications.Int J Mol Sci. 2023 Mar 22;24(6):5994. doi: 10.3390/ijms24065994. Int J Mol Sci. 2023. PMID: 36983063 Free PMC article. Review.
-
From antibodies to nanobodies: The next frontier in cancer theranostics for solid tumors.Adv Protein Chem Struct Biol. 2025;144:287-329. doi: 10.1016/bs.apcsb.2024.10.014. Epub 2025 Jan 30. Adv Protein Chem Struct Biol. 2025. PMID: 39978969 Review.
-
Nanobodies as versatile tools: A focus on targeted tumor therapy, tumor imaging and diagnostics.Hum Antibodies. 2020;28(4):259-272. doi: 10.3233/HAB-200425. Hum Antibodies. 2020. PMID: 32831197 Review.
-
Nanobodies as molecular imaging probes.Free Radic Biol Med. 2022 Mar;182:260-275. doi: 10.1016/j.freeradbiomed.2022.02.031. Epub 2022 Feb 28. Free Radic Biol Med. 2022. PMID: 35240292 Review.
Cited by
-
Exploring the therapeutic use and outcome of antibody-drug conjugates in ovarian cancer treatment.Oncogene. 2025 Jul;44(28):2343-2356. doi: 10.1038/s41388-025-03448-3. Epub 2025 May 27. Oncogene. 2025. PMID: 40514426 Free PMC article. Review.
-
GRP78 Nanobody-Directed Immunotoxin Activates Innate Immunity Through STING Pathway to Synergize Tumor Immunotherapy.Adv Sci (Weinh). 2025 May;12(19):e2408086. doi: 10.1002/advs.202408086. Epub 2025 Mar 26. Adv Sci (Weinh). 2025. PMID: 40135833 Free PMC article.
-
Aptamers and Nanobodies as New Bioprobes for SARS-CoV-2 Diagnostic and Therapeutic System Applications.Biosensors (Basel). 2024 Mar 15;14(3):146. doi: 10.3390/bios14030146. Biosensors (Basel). 2024. PMID: 38534253 Free PMC article. Review.
References
-
- Maeda R., Fujita J., Konishi Y., Kazuma Y., Yamazaki H., Anzai I., Watanabe T., Yamaguchi K., Kasai K., Nagata K., et al. A panel of nanobodies recognizing conserved hidden clefts of all SARS-CoV-2 spike variants including Omicron. Commun. Biol. 2022;5:669. doi: 10.1038/s42003-022-03630-3. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

