Efficient Radial-Shell Model for 3D Tumor Spheroid Dynamics with Radiotherapy
- PMID: 38067348
- PMCID: PMC10705470
- DOI: 10.3390/cancers15235645
Efficient Radial-Shell Model for 3D Tumor Spheroid Dynamics with Radiotherapy
Abstract
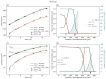
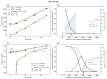
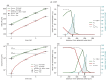
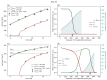
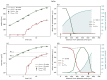
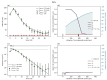
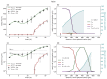
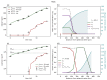
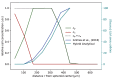
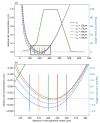
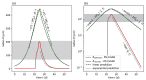
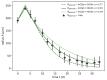
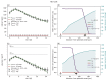
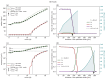
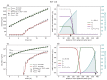
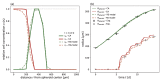
Understanding the complex dynamics of tumor growth to develop more efficient therapeutic strategies is one of the most challenging problems in biomedicine. Three-dimensional (3D) tumor spheroids, reflecting avascular microregions within a tumor, are an advanced in vitro model system to assess the curative effect of combinatorial radio(chemo)therapy. Tumor spheroids exhibit particular crucial pathophysiological characteristics such as a radial oxygen gradient that critically affect the sensitivity of the malignant cell population to treatment. However, spheroid experiments remain laborious, and determining long-term radio(chemo)therapy outcomes is challenging. Mathematical models of spheroid dynamics have the potential to enhance the informative value of experimental data, and can support study design; however, they typically face one of two limitations: while non-spatial models are computationally cheap, they lack the spatial resolution to predict oxygen-dependent radioresponse, whereas models that describe spatial cell dynamics are computationally expensive and often heavily parameterized, impeding the required calibration to experimental data. Here, we present an effectively one-dimensional mathematical model based on the cell dynamics within and across radial spheres which fully incorporates the 3D dynamics of tumor spheroids by exploiting their approximate rotational symmetry. We demonstrate that this radial-shell (RS) model reproduces experimental spheroid growth curves of several cell lines with and without radiotherapy, showing equal or better performance than published models such as 3D agent-based models. Notably, the RS model is sufficiently efficient to enable multi-parametric optimization within previously reported and/or physiologically reasonable ranges based on experimental data. Analysis of the model reveals that the characteristic change of dynamics observed in experiments at small spheroid volume originates from the spatial scale of cell interactions. Based on the calibrated parameters, we predict the spheroid volumes at which this behavior should be observable. Finally, we demonstrate how the generic parameterization of the model allows direct parameter transfer to 3D agent-based models.
Keywords: 3D growth; cellular automaton; growth curve; minimal model; radial shell model; radiation therapy; simulation; spatio-temporal mathematical modelling; spheroids; systems biology; tumor relapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Image segmentation of treated and untreated tumor spheroids by fully convolutional networks.Gigascience. 2025 Jan 6;14:giaf027. doi: 10.1093/gigascience/giaf027. Gigascience. 2025. PMID: 40331344 Free PMC article.
-
Formation and Growth of Co-Culture Tumour Spheroids: New Compartment-Based Mathematical Models and Experiments.Bull Math Biol. 2023 Dec 13;86(1):8. doi: 10.1007/s11538-023-01229-1. Bull Math Biol. 2023. PMID: 38091169
-
Mathematical modelling reveals cellular dynamics within tumour spheroids.PLoS Comput Biol. 2020 Aug 18;16(8):e1007961. doi: 10.1371/journal.pcbi.1007961. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32810174 Free PMC article.
-
Growth and cellular characteristics of multicell spheroids.Recent Results Cancer Res. 1984;95:24-49. doi: 10.1007/978-3-642-82340-4_2. Recent Results Cancer Res. 1984. PMID: 6396760 Review.
-
An Introduction to Image-Based Systems Biology of Multicellular Spheroids for Experimentalists and Theoreticians.In: Husi H, editor. Computational Biology [Internet]. Brisbane (AU): Codon Publications; 2019 Nov 21. Chapter 1. In: Husi H, editor. Computational Biology [Internet]. Brisbane (AU): Codon Publications; 2019 Nov 21. Chapter 1. PMID: 31815399 Free Books & Documents. Review.
Cited by
-
Image segmentation of treated and untreated tumor spheroids by fully convolutional networks.Gigascience. 2025 Jan 6;14:giaf027. doi: 10.1093/gigascience/giaf027. Gigascience. 2025. PMID: 40331344 Free PMC article.
-
A Hydrogel for Nitric Oxide Sensitization Chemotherapy Mediated by Tumor Microenvironment Changes in 3D Spheroids and Breast Tumor Models.Curr Pharm Des. 2025;31(15):1227-1238. doi: 10.2174/0113816128348357241209050425. Curr Pharm Des. 2025. PMID: 39819416
-
Transient flow-induced deformation of cancer cells in microchannels: a general computational model and experiments.Biomech Model Mechanobiol. 2025 Apr;24(2):489-506. doi: 10.1007/s10237-024-01920-9. Epub 2025 Feb 2. Biomech Model Mechanobiol. 2025. PMID: 39893594 Free PMC article.
References
-
- Leek R., Grimes D.R., Harris A.L., McIntyre A. Methods: Using Three-Dimensional Culture (Spheroids) as an In Vitro Model of Tumour Hypoxia. In: Koumenis C., Coussens L.M., Giaccia A., Hammond E., editors. Proceedings of the Tumor Microenvironment. Springer International Publishing; Berlin/Heidelberg, Germany: 2016. pp. 167–196. Advances in Experimental Medicine and Biology. - DOI - PubMed
Grants and funding
- 100382145/the EU, the European Social Fund (ESF) and by tax funds on the basis of the budget passed by the Saxon state parliament
- FORZUG II TP 3/Sächsisches Staatsministerium für Wissenschaft und Kunst (SMWK)
- BMBF 16dkwn001a/b/Bundesministerium für Bildung und Forschung
- AL1705/11/DFG
- 491382348/Open Access Publication Fund of Hochschule für Technik und Wirtschaft Dresden - University of Applied Sciences and by Deutsche Forschungsgemeinschaft
LinkOut - more resources
Full Text Sources

