Role of Vitamin C in Targeting Cancer Stem Cells and Cellular Plasticity
- PMID: 38067361
- PMCID: PMC10705783
- DOI: 10.3390/cancers15235657
Role of Vitamin C in Targeting Cancer Stem Cells and Cellular Plasticity
Abstract
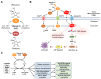
Vitamin C (VC) is an essential nutrient that is vital for maintaining cellular physiology. Interestingly, it functions as either an antioxidant or a pro-oxidant, depending on the concentration used. At high-doses, VC selectively targets various cancer cell types through its pro-oxidant action, while at low-doses, VC enhances anti-tumor immunity by acting as an antioxidant. This versatility makes VC a promising anti-tumor agent for both standalone and combination therapies. Tumors consist of diverse cancer cell subtypes with distinct phenotypic and functional characteristics. In particular, cancer stem cells (CSCs), which are self-renewing multi-potent cells, are responsible for tumor recurrence, metastasis, chemoresistance, and heightened mortality. CSCs are often associated with the epithelial-mesenchymal transition (EMT), which confers increased motility and invasive capabilities that are characteristic of malignant and drug-resistant cells. Thus, eradicating CSC populations is crucial and has led to extensive efforts aimed at identifying medicines that can target them. Recent studies suggest that VC can selectively target CSCs via epigenetic and metabolic pathways in various cancers. Here, we highlight recent progress that has been made in understanding how VC effectively targets CSC evolution, providing a rationale for the use of VC either alone or in combination with other treatments to improve outcomes.
Keywords: cancer stem cells; epithelial–mesenchymal transition; vitamin C.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
Potential Role of the Circadian Clock in the Regulation of Cancer Stem Cells and Cancer Therapy.Int J Mol Sci. 2022 Nov 16;23(22):14181. doi: 10.3390/ijms232214181. Int J Mol Sci. 2022. PMID: 36430659 Free PMC article. Review.
-
Targeting redox regulation and autophagy systems in cancer stem cells.Clin Exp Med. 2023 Sep;23(5):1405-1423. doi: 10.1007/s10238-022-00955-5. Epub 2022 Dec 6. Clin Exp Med. 2023. PMID: 36473988 Review.
-
Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma.J Hematol Oncol. 2016 Aug 30;9(1):74. doi: 10.1186/s13045-016-0307-9. J Hematol Oncol. 2016. PMID: 27578206 Free PMC article. Review.
-
Basal/HER2 breast carcinomas: integrating molecular taxonomy with cancer stem cell dynamics to predict primary resistance to trastuzumab (Herceptin).Cell Cycle. 2013 Jan 15;12(2):225-45. doi: 10.4161/cc.23274. Epub 2012 Jan 15. Cell Cycle. 2013. PMID: 23255137 Free PMC article.
-
Epithelial-mesenchymal transition: a hallmark in pancreatic cancer stem cell migration, metastasis formation, and drug resistance.J Cancer Metastasis Treat. 2020;6:36. doi: 10.20517/2394-4722.2020.55. Epub 2020 Sep 27. J Cancer Metastasis Treat. 2020. PMID: 34841087 Free PMC article.
Cited by
-
Core Molecular Clock Factors Regulate Osteosarcoma Stem Cell Survival and Behavior via CSC/EMT Pathways and Lipid Droplet Biogenesis.Cells. 2025 Mar 31;14(7):517. doi: 10.3390/cells14070517. Cells. 2025. PMID: 40214471 Free PMC article.
-
Redox-active vitamin C suppresses human osteosarcoma growth by triggering intracellular ROS-iron-calcium signaling crosstalk and mitochondrial dysfunction.Redox Biol. 2024 Sep;75:103288. doi: 10.1016/j.redox.2024.103288. Epub 2024 Jul 26. Redox Biol. 2024. PMID: 39083898 Free PMC article.
-
Association of circulating vitamin levels with thyroid diseases: a Mendelian randomization study.Front Endocrinol (Lausanne). 2024 Jun 11;15:1360851. doi: 10.3389/fendo.2024.1360851. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38919472 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

