Estimating the Time Toxicity of Contemporary Systemic Treatment Regimens for Advanced Esophageal and Gastric Cancers
- PMID: 38067380
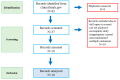
- PMCID: PMC10705178
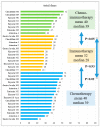
- DOI: 10.3390/cancers15235677
Estimating the Time Toxicity of Contemporary Systemic Treatment Regimens for Advanced Esophageal and Gastric Cancers
Abstract
(1) Background: The purpose of this study was to evaluate the time toxicity, or time spent in health care, of immunotherapy- versus chemotherapy-based regimens for metastatic esophageal and gastric cancers. (2) Methods: A literature search was conducted, and 18 phase III clinical trials of immune checkpoint inhibitors were selected for analysis. Health care days were calculated based on the number of days associated with receiving therapy and the adverse events reported in the clinical trials. Both the number of health care days and the median overall survival were compared among chemotherapy-only, immunotherapy-only, and chemo-immunotherapy regimens across this cohort of drug registration trials. (3) Results: The estimated median number of health care days was 37 (range of 7-52) days, or 1.2 (range of 0.2-1.7) months, compared to a median survival of 10.2 months across these 18 studies. For the chemotherapy-only regimens, the median number of health care days was 39 (range of 21-51) days, and for chemo-immunotherapy, it was 39 (range of 30-52) days. The immunotherapy-only regimens had fewer days, a median of 28 (range of 24-41), p < 0.05, compared to the other two arms. (4) Conclusions: The chemo-immunotherapy regimens did not add time toxicity compared to chemotherapy alone. The immunotherapy-only regimens had lower time toxicity compared to chemotherapy alone. In the setting of decreased time toxicity and improved overall survival, further development of immunotherapy-based regimens could improve outcomes in advanced esophageal and gastric cancers.
Keywords: clinical trials; esophageal cancer; gastric cancer; hospital days; time toxicities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The efficacy and safety of first‑line anti‑PD‑1/PD‑L1 immunotherapy for gastric esophageal cancer: A systematic review and meta‑analysis of phase III randomized controlled trials.Exp Ther Med. 2023 Mar 28;25(5):216. doi: 10.3892/etm.2023.11915. eCollection 2023 May. Exp Ther Med. 2023. PMID: 37123204 Free PMC article.
-
Efficacy and safety of PD-1/PD-L1 inhibitor combined with chemotherapy versus chemotherapy alone in the treatment of advanced gastric or gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis.Front Oncol. 2023 Apr 11;13:1077675. doi: 10.3389/fonc.2023.1077675. eCollection 2023. Front Oncol. 2023. PMID: 37114136 Free PMC article.
-
Efficacy and safety of immunochemotherapy, immunotherapy, chemotherapy, and targeted therapy as first-line treatment for advanced and metastatic esophageal cancer: a systematic review and network meta-analysis.Lancet Reg Health West Pac. 2023 Jul 6;38:100841. doi: 10.1016/j.lanwpc.2023.100841. eCollection 2023 Sep. Lancet Reg Health West Pac. 2023. PMID: 37457900 Free PMC article.
-
[Safety and efficacy of laparoscopic surgery in locally advanced gastric cancer patients with neoadjuvant chemotherapy combined with immunotherapy].Zhonghua Wei Chang Wai Ke Za Zhi. 2023 Jan 25;26(1):84-92. doi: 10.3760/cma.j.cn441530-20220616-00265. Zhonghua Wei Chang Wai Ke Za Zhi. 2023. PMID: 36650004 Chinese.
-
Immune Therapeutics in the Treatment of Advanced Gastric and Esophageal Cancer.Anticancer Res. 2018 Oct;38(10):5569-5580. doi: 10.21873/anticanres.12891. Anticancer Res. 2018. PMID: 30275174 Review.
Cited by
-
Trends in complexity of single-agent and combination therapies for solid tumor cancers approved by the US Food and Drug Administration.Oncologist. 2025 Mar 10;30(3):oyae302. doi: 10.1093/oncolo/oyae302. Oncologist. 2025. PMID: 39548871 Free PMC article.
-
Time's up: the urgency to investigate time toxicity in patients with genitourinary malignancies.Ther Adv Med Oncol. 2024 Dec 11;16:17588359241305088. doi: 10.1177/17588359241305088. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39664299 Free PMC article. Review.
-
Patients' perspectives on oral decitabine/cedazuridine for the treatment of myelodysplastic syndromes/neoplasms.Ther Adv Hematol. 2024 Jul 30;15:20406207241257313. doi: 10.1177/20406207241257313. eCollection 2024. Ther Adv Hematol. 2024. PMID: 39091323 Free PMC article.
-
Implementation of a Traceback Testing Program for Ovarian Cancer: Findings from the FACTS Study.Cancers (Basel). 2025 Mar 29;17(7):1154. doi: 10.3390/cancers17071154. Cancers (Basel). 2025. PMID: 40227675 Free PMC article.
-
Telehealth and Health Care Contact Days Among Patients With Advanced Cancer After COVID-19.JAMA Netw Open. 2025 Jun 2;8(6):e2516762. doi: 10.1001/jamanetworkopen.2025.16762. JAMA Netw Open. 2025. PMID: 40540268 Free PMC article.
References
LinkOut - more resources
Full Text Sources