Metronomic Temozolomide (mTMZ) and Bevacizumab-The Safe and Effective Frontier for Treating Metastatic Neuroendocrine Tumors (NETs): A Single-Center Experience
- PMID: 38067391
- PMCID: PMC10705735
- DOI: 10.3390/cancers15235688
Metronomic Temozolomide (mTMZ) and Bevacizumab-The Safe and Effective Frontier for Treating Metastatic Neuroendocrine Tumors (NETs): A Single-Center Experience
Abstract
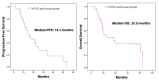
Addressing the persistent challenges in treating metastatic neuroendocrine tumors (NETs) demands ongoing refinement and innovation in therapeutic strategies. This study investigates the potential advantages of combining metronomic temozolomide (mTMZ) with bevacizumab for patients diagnosed with metastatic NETs, particularly focusing on those with a Ki-67 index under 55%. Data from 30 patients were analyzed, using key performance indicators such as progression-free survival (PFS), overall survival (OS), and response rates to therapy, to gauge the treatment's efficacy. The results were encouraging: the median PFS recorded was 16.3 months, and the OS was 25.9 months. The disease control rate (DCR) reached an impressive 86.7%, and the objective response rate (ORR) stood at 63.3%. The treatment regimen was well-tolerated, with no reported instances of grade 4 toxicities. Such a safety profile indicates that this regimen may be particularly advantageous for older, fragile patients who might struggle with conventional dosage levels. These initial findings suggest that the mTMZ and bevacizumab combination could potentially rival the conventional temozolomide-capecitabine therapy in managing metastatic NETs. We aimed to meticulously assess the efficacy of the mTMZ and bevacizumab combination in treating metastatic NETs. Given the initial promising results, a more conclusive understanding of its efficacy will require further research through larger, multicenter prospective clinical trials.
Keywords: CAPTEM; bevacizumab; mTMZ; metronomic temozolomide; neuroendocrine neoplasia; neuroendocrine tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Temozolomide Alone or Combined with Capecitabine for the Treatment of Metastatic Neuroendocrine Neoplasia: A "Real-World" Data Analysis.Neuroendocrinology. 2021;111(9):895-906. doi: 10.1159/000513218. Epub 2020 Nov 20. Neuroendocrinology. 2021. PMID: 33221806
-
The Role of Capecitabine/Temozolomide in Metastatic Neuroendocrine Tumors.Oncologist. 2016 Jun;21(6):671-5. doi: 10.1634/theoncologist.2015-0470. Epub 2016 May 25. Oncologist. 2016. PMID: 27226359 Free PMC article.
-
Efficacy of Capecitabine and Temozolomide Regimen in Neuroendocrine Tumors: Data From the Turkish Oncology Group.Oncologist. 2023 Oct 3;28(10):875-884. doi: 10.1093/oncolo/oyad257. Oncologist. 2023. PMID: 37676712 Free PMC article.
-
Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: A meta-analysis.Medicine (Baltimore). 2018 Oct;97(41):e12784. doi: 10.1097/MD.0000000000012784. Medicine (Baltimore). 2018. PMID: 30313101 Free PMC article. Review.
-
Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs): A Systematic Review and Pooled Analysis.Cancer Manag Res. 2022 Dec 21;14:3507-3523. doi: 10.2147/CMAR.S372776. eCollection 2022. Cancer Manag Res. 2022. PMID: 36575665 Free PMC article. Review.
References
-
- Pavel M., Öberg K., Falconi M., Krenning E.P., Sundin A., Perren A., Berruti A., ESMO Guidelines Committee Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:844–860. doi: 10.1016/j.annonc.2020.03.304. - DOI - PubMed
LinkOut - more resources
Full Text Sources

