Unveiling the Immunogenicity of Ovarian Tumors as the Crucial Catalyst for Therapeutic Success
- PMID: 38067396
- PMCID: PMC10705691
- DOI: 10.3390/cancers15235694
Unveiling the Immunogenicity of Ovarian Tumors as the Crucial Catalyst for Therapeutic Success
Abstract
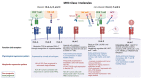
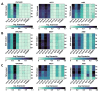
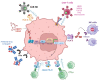
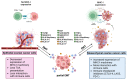
Epithelial ovarian cancer (EOC) is the most lethal gynecologic cancer. The disease is often diagnosed after wide-spread dissemination, and the standard treatment combines aggressive surgery with platinum-based chemotherapy; however, most patients experience relapse in the form of peritoneal carcinomatosis, resulting in a 5-year mortality below 45%. There is clearly a need for the development of novel treatments and cancer immunotherapies offering a different approach. Immunotherapies have demonstrated their efficacy in many types of cancers; however, only <15% of EOC patients show any evidence of response. One of the main barriers behind the poor therapeutic outcome is the reduced expression of Major Histocompatibility Complexes class I (MHC I) which occurs in approximately 60% of EOC cases. This review aims to gather and enhance our current understanding of EOC, focusing on its distinct cancer characteristics related to MHC I expression, immunogenicity, antigen presentation, epithelial-to-mesenchymal transition, and various ongoing immunotherapeutic strategies designed to stimulate antitumor immunity.
Keywords: EMT; classic HLA I; non-classic HLA I; ovarian cancer; tumor immunogenicity; tumor-associated antigens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination.Front Endocrinol (Lausanne). 2022 Apr 28;13:886533. doi: 10.3389/fendo.2022.886533. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574025 Free PMC article. Review.
-
Loss of 4.1N in epithelial ovarian cancer results in EMT and matrix-detached cell death resistance.Protein Cell. 2021 Feb;12(2):107-127. doi: 10.1007/s13238-020-00723-9. Epub 2020 May 25. Protein Cell. 2021. PMID: 32448967 Free PMC article.
-
Combined chemotherapy and allogeneic human Vγ9Vδ2 T lymphocyte-immunotherapies efficiently control the development of human epithelial ovarian cancer cells in vivo.Oncoimmunology. 2019 Aug 28;8(11):e1649971. doi: 10.1080/2162402X.2019.1649971. eCollection 2019. Oncoimmunology. 2019. PMID: 31646097 Free PMC article.
-
The mannose receptor LY75 (DEC205/CD205) modulates cellular phenotype and metastatic potential of ovarian cancer cells.Oncotarget. 2016 Mar 22;7(12):14125-42. doi: 10.18632/oncotarget.7288. Oncotarget. 2016. PMID: 26871602 Free PMC article.
-
Nanoparticle Delivery of TWIST Small Interfering RNA and Anticancer Drugs: A Therapeutic Approach for Combating Cancer.Enzymes. 2018;44:83-101. doi: 10.1016/bs.enz.2018.08.004. Epub 2018 Oct 5. Enzymes. 2018. PMID: 30360816 Review.
Cited by
-
Immunotherapy for Platinum-Resistant Ovarian Cancer as a Glimmer of Hope.Cells. 2025 Jun 29;14(13):995. doi: 10.3390/cells14130995. Cells. 2025. PMID: 40643516 Free PMC article. Review.
-
Inflammation and Immune Escape in Ovarian Cancer: Pathways and Therapeutic Opportunities.J Inflamm Res. 2025 Jan 21;18:895-909. doi: 10.2147/JIR.S503479. eCollection 2025. J Inflamm Res. 2025. PMID: 39867950 Free PMC article. Review.
-
A patient stratification signature mirrors the immunogenic potential of high grade serous ovarian cancers.J Transl Med. 2024 Nov 20;22(1):1048. doi: 10.1186/s12967-024-05846-9. J Transl Med. 2024. PMID: 39568014 Free PMC article.
-
Ovarian cancer: Diagnosis and treatment strategies (Review).Oncol Lett. 2024 Jul 18;28(3):441. doi: 10.3892/ol.2024.14574. eCollection 2024 Sep. Oncol Lett. 2024. PMID: 39099583 Free PMC article. Review.
References
-
- Cruz-Tapias P., Castiblanco J., Anaya J.-M. Autoimmunity: From Bench to Bedside [Internet] El Rosario University Press; Bogotá, Colombia: 2013. HLA Association with Autoimmune Diseases. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

