Analysis of Health-Related Quality of Life Reporting in Phase III RCTs of Advanced Genitourinary Tumors
- PMID: 38067406
- PMCID: PMC10705354
- DOI: 10.3390/cancers15235703
Analysis of Health-Related Quality of Life Reporting in Phase III RCTs of Advanced Genitourinary Tumors
Abstract
Background: As recommended in the European Society for Medical Oncology (ESMO) guidelines, assessment of health-related quality of life (HRQoL) should be a relevant endpoint in randomized controlled trials (RCTs) testing new anticancer therapies. However, previous publications by our group and others revealed a frequent underestimation and underreporting of HRQoL results in publication of RCTs in oncology. Herein, we systematically reviewed HRQoL reporting in RCTs testing new treatments in advanced prostate, kidney and urothelial cancers and published between 2010 and 2022.
Methods: We searched PubMed RCTs testing novel therapies in genitourinary (GU) cancers and published in fifteen selected journals (Annals of Oncology, BMC Cancer, British Journal of Cancer, Cancer Discovery, Clinical Cancer Research, Clinical Genitourinary cancer, European Journal of Cancer, European Urology, European Urology Oncology, JAMA, JAMA Oncology, Journal of clinical Oncology, Lancet, Lancet Oncology and The New England Journal of Medicine). We excluded trials investigating exclusively best supportive care or behavioral intervention, as well as subgroup or post hoc analyses of previously published trials. For each RCT, we investigated whether HRQoL assessment was performed by protocol and if results were reported in the primary manuscript or in a secondary publication.
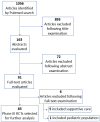
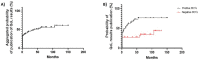
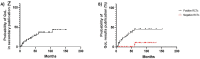
Results: We found 85 eligible trials published between 2010 and 2022. Only 1/85 RCTs (1.2%) included HRQoL among primary endpoints. Of note, 25/85 (29.4%) RCTs did not include HRQoL among study endpoints. HRQoL results were non-disclosed in 56/85 (65.9%) primary publications. Only 18/85 (21.2%) publications fulfilled at least one item of the CONSORT-PRO checklist. Furthermore, 14/46 (30.4%) RCTs in prostate cancer, 12/25 (48%) in kidney cancer and 3/14 (21.4%) in urothelial cancer reported HRQoL data in primary publications. Next, HRQoL data were disclosed in primary manuscripts of 12/32 (37.5%), 5/13 (38.5%), 5/16 (31.3%) and 5/15 (33.3%) trials evaluating target therapies, chemotherapy, immunotherapy and new hormonal agents, respectively. Next, we found that HRQoL data were reported in 16/42 (38%) and in 13/43 (30.2%) positive and negative trials, respectively. Finally, the rate of RCTs reporting HRQoL results in primary or secondary publications was 55.3% (n = 47/85).
Conclusions: Our analysis revealed a relevant underreporting of HRQoL in RCTs in advanced GU cancers. These results highlight the need to dedicate more attention to HRQoL in RCTs to fully assess the value of new anticancer treatments.
Keywords: CONSORT-PRO; genitourinary cancers; health-related quality of life; patient-reported outcomes; randomized controlled trials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Patient-Reported Outcomes in Phase 3 Clinical Trials for Blood Cancers: A Systematic Review.JAMA Netw Open. 2024 Jun 3;7(6):e2414425. doi: 10.1001/jamanetworkopen.2024.14425. JAMA Netw Open. 2024. PMID: 38829615 Free PMC article.
-
Reporting Quality of Randomized Controlled Trials of Periodontal Diseases in Journal Abstracts-A Cross-sectional Survey and Bibliometric Analysis.J Evid Based Dent Pract. 2018 Jun;18(2):130-141.e22. doi: 10.1016/j.jebdp.2017.08.005. Epub 2017 Sep 21. J Evid Based Dent Pract. 2018. PMID: 29747793
-
Health-related quality of life is underestimated and underreported in phase III clinical trials in NSCLC.Lung Cancer. 2022 Dec;174:36-44. doi: 10.1016/j.lungcan.2022.10.003. Epub 2022 Oct 19. Lung Cancer. 2022. PMID: 36302311 Clinical Trial.
-
Associations between safety, tolerability, and toxicity and the reporting of health-related quality of life in phase III randomized trials in common solid tumors.Cancer Med. 2020 Nov;9(21):7888-7895. doi: 10.1002/cam4.3390. Epub 2020 Sep 4. Cancer Med. 2020. PMID: 32886422 Free PMC article.
-
Reporting of health-related quality of life (HRQOL) data in oncology trials: a comparison of the European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy-General (FACT-G).Qual Life Res. 2014 Apr;23(3):971-6. doi: 10.1007/s11136-013-0534-2. Epub 2013 Oct 5. Qual Life Res. 2014. PMID: 24097080 Review.
Cited by
-
CRO-67 has anti-cancer activity in pancreatic tumor cells and stromal cancer-associated fibroblasts.Sci Rep. 2025 Jul 8;15(1):24488. doi: 10.1038/s41598-025-09411-2. Sci Rep. 2025. PMID: 40628852 Free PMC article.
-
Synchronous surgery combined preoperative chemotherapy benefits patients suffering pancreatic ductal adenocarcinoma with liver metastases: a systematic review and meta-analysis.Sci Rep. 2025 Aug 4;15(1):28403. doi: 10.1038/s41598-025-13811-9. Sci Rep. 2025. PMID: 40759716 Free PMC article.
References
-
- Choueiri T.K., Powles T., Burotto M., Escudier B., Bourlon M.T., Zurawski B., CheckMate 9ER Investigators. Oyervides Juárez V.M., Hsieh J.J., Basso U., et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. - DOI - PMC - PubMed
-
- Motzer R.J., Tannir N.M., McDermott D.F., Aren Frontera O., Melichar B., Choueiri T.K., CheckMate 214 Investigators. Plimack E.R., Barthélémy P., Porta C., et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

