Design and Synthesis of Novel α-Methylchalcone Derivatives, Anti-Cervical Cancer Activity, and Reversal of Drug Resistance in HeLa/DDP Cells
- PMID: 38067428
- PMCID: PMC10707934
- DOI: 10.3390/molecules28237697
Design and Synthesis of Novel α-Methylchalcone Derivatives, Anti-Cervical Cancer Activity, and Reversal of Drug Resistance in HeLa/DDP Cells
Abstract
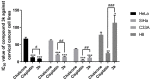
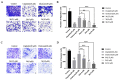
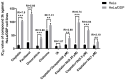
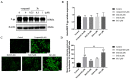
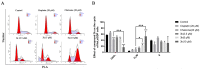
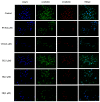
In this study, a collection of newly developed α-methylchalcone derivatives were synthesized and assessed for their inhibitory potential against human cervical cancer cell lines (HeLa, SiHa, and C33A) as well as normal human cervical epithelial cells (H8). Notably, compound 3k exhibited substantial inhibitory effects on both HeLa and HeLa/DDP cells while demonstrating lower toxicity toward H8 cells. Furthermore, the compound 3k was found to induce apoptosis in both HeLa and HeLa/DDP cells while also inhibiting the G2/M phase, resulting in a decrease in the invasion and migration capabilities of these cells. When administered alongside cisplatin, 3k demonstrated a significant reduction in the resistance of HeLa/DDP cells to cisplatin, as evidenced by a decrease in the resistance index (RI) value from 7.90 to 2.10. Initial investigations into the underlying mechanism revealed that 3k did not impact the expression of P-gp but instead facilitated the accumulation of rhodamine 123 in HeLa/DDP cells. The results obtained from CADD docking analysis demonstrated that 3k exhibits stable binding to microtubule proteins and P-gp targets, forming hydrogen bonding interaction forces. Immunofluorescence analysis further revealed that 3k effectively decreased the fluorescence intensity of α and β microtubules in HeLa and HeLa/DDP cells, resulting in disruptions in cell morphology, reduction in cell numbers, nucleus coagulation, and cell rupture. Additionally, Western blot analysis indicated that 3k significantly reduced the levels of polymerized α and β microtubule proteins in both HeLa and HeLa/DDP cell lines while concurrently increasing the expression of dissociated α and β microtubule proteins. The aforementioned findings indicate a potential correlation between the inhibitory effects of 3k on HeLa and HeLa/DDP cells and its ability to inhibit tubulin and P-gp.
Keywords: P-gp; cervical cancer; cisplatin resistance; tubulin; α-methyl chalcone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Design and synthesis of novel imidazole-chalcone derivatives as microtubule protein polymerization inhibitors to treat cervical cancer and reverse cisplatin resistance.Bioorg Chem. 2024 Jun;147:107310. doi: 10.1016/j.bioorg.2024.107310. Epub 2024 Apr 4. Bioorg Chem. 2024. PMID: 38583249
-
Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives.Molecules. 2023 Jun 3;28(11):4537. doi: 10.3390/molecules28114537. Molecules. 2023. PMID: 37299013 Free PMC article.
-
TMEM45A Affects Proliferation, Apoptosis, Epithelial-Mesenchymal Transition, Migration, Invasion and Cisplatin Resistance of HPV-Positive Cervical Cancer Cell Lines.Biochem Genet. 2022 Feb;60(1):173-190. doi: 10.1007/s10528-021-10094-3. Epub 2021 Jun 18. Biochem Genet. 2022. PMID: 34143331
-
Design, Synthesis, and In vitro Anti-cervical Cancer Activity of a Novel MDM2-p53 Inhibitor Based on a Chalcone Scaffold.Anticancer Agents Med Chem. 2024;24(6):423-435. doi: 10.2174/0118715206274066231220071557. Anticancer Agents Med Chem. 2024. PMID: 38204258
-
Cellular landscaping of cisplatin resistance in cervical cancer.Biomed Pharmacother. 2022 Sep;153:113345. doi: 10.1016/j.biopha.2022.113345. Epub 2022 Jul 8. Biomed Pharmacother. 2022. PMID: 35810692 Review.
Cited by
-
In Silico and In Vitro Analyses of Strawberry-Derived Extracts in Relation to Key Compounds' Metabolic and Anti-Tumor Effects.Int J Mol Sci. 2025 Apr 8;26(8):3492. doi: 10.3390/ijms26083492. Int J Mol Sci. 2025. PMID: 40331930 Free PMC article.
References
-
- Liu X., Xing Y., Li M., Zhang Z., Wang J., Ri M., Jin C., Xu G., Piao L., Jin H., et al. Licochalcone a inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the nf-κb and ras/raf/mek pathways. J. Ethnopharmacol. 2021;273:113989. doi: 10.1016/j.jep.2021.113989. - DOI - PubMed
-
- Doherty B., Lawlor D., Gillet J.P., Gottesman M., O’Leary J.J., Stordal B. Collateral sensitivity to cisplatin in kb-8-5-11 drug-resistant cancer cells. Anticancer Res. 2014;34:503–507. - PubMed
MeSH terms
Substances
Grants and funding
- 81960625/the National Natural Science Foundation of China
- 82160654/the National Natural Science Foundation of China
- No. XJDX1713/The Project of Xinjiang Key Laboratory of Active Components of Natural Medicine and Drug Release Technology
- XJ2022G168/Xinjiang Uygur Autonomous Region Postgraduate Research Innovation Project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

