Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis
- PMID: 38067436
- PMCID: PMC10708292
- DOI: 10.3390/molecules28237705
Antifungal Constituents of Piper crocatum and Their Activities as Ergosterol Biosynthesis Inhibitors Discovered via In Silico Study Using ADMET and Drug-Likeness Analysis
Abstract
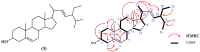
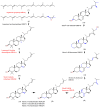
Along with the increasing resistance of Candida spp. to some antibiotics, it is necessary to find new antifungal drugs, one of which is from the medicinal plant Red Betel (Piper crocatum). The purpose of this research is to isolate antifungal constituents from P. crocatum and evaluate their activities as ergosterol biosynthesis inhibitors via an in silico study of ADMET and drug-likeness analysis. Two new active compounds 1 and 2 and a known compound 3 were isolated, and their structures were determined using spectroscopic methods, while their bioactivities were evaluated via in vitro and in silico studies, respectively. Antifungal compound 3 was the most active compared to 1 and 2 with zone inhibition values of 14.5, 11.9, and 13.0 mm, respectively, at a concentration of 10% w/v, together with MIC/MFC at 0.31/1.2% w/v. Further in silico study demonstrated that compound 3 had a stronger ΔG than the positive control and compounds 1 and 2 with -11.14, -12.78, -12.00, and -6.89 Kcal/mol against ERG1, ERG2, ERG11, and ERG24, respectively, and also that 3 had the best Ki with 6.8 × 10-3, 4 × 10-4, 1.6 × 10-3, and 8.88 μM. On the other hand, an ADMET analysis of 1-3 met five parameters, while 1 had one violation of Ro5. Based on the research data, the promising antifungal constituents of P. crocatum allow P. crocatum to be proposed as a new antifungal candidate to treat and cure infections due to C. albicans.
Keywords: ADMET; Piper crocatum; antifungal; drug-likeness analysis; ergosterol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Phytochemical profiling of Piper crocatum and its antifungal mechanism action as Lanosterol 14 alpha demethylase CYP51 inhibitor: a review.F1000Res. 2022 Sep 28;11:1115. doi: 10.12688/f1000research.125645.3. eCollection 2022. F1000Res. 2022. PMID: 37151610 Free PMC article. Review.
-
In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species.Sci Rep. 2017 Dec 5;7(1):16942. doi: 10.1038/s41598-017-17239-8. Sci Rep. 2017. PMID: 29209074 Free PMC article.
-
New miconazole-based azoles derived from eugenol show activity against Candida spp. and Cryptococcus gattii by inhibiting the fungal ergosterol biosynthesis.Eur J Med Chem. 2023 Aug 5;256:115436. doi: 10.1016/j.ejmech.2023.115436. Epub 2023 May 1. Eur J Med Chem. 2023. PMID: 37146343
-
Synergistic antifungal activity and potential mechanism of action of a glycolipid-like compound produced by Streptomyces blastmyceticus S108 against Candida clinical isolates.J Appl Microbiol. 2023 Nov 1;134(11):lxad246. doi: 10.1093/jambio/lxad246. J Appl Microbiol. 2023. PMID: 37884451
-
Molecular epidemiology, antifungal susceptibility, and ERG11 gene mutation of Candida species isolated from vulvovaginal candidiasis: Comparison between recurrent and non-recurrent infections.Microb Pathog. 2022 Sep;170:105696. doi: 10.1016/j.micpath.2022.105696. Epub 2022 Jul 31. Microb Pathog. 2022. PMID: 35921954 Review.
Cited by
-
Anti-Infection of Oral Microorganisms from Herbal Medicine of Piper crocatum Ruiz & Pav.Drug Des Devel Ther. 2024 Jun 25;18:2531-2553. doi: 10.2147/DDDT.S453375. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38952486 Free PMC article. Review.
-
Density Functional Theory, Molecular Docking Study, and In Vitro Antioxidant Activity of Cinnamic Acid Isolated From Piper betle Leaves.Biochem Res Int. 2025 Jun 17;2025:1691257. doi: 10.1155/bri/1691257. eCollection 2025. Biochem Res Int. 2025. PMID: 40556747 Free PMC article.
-
Potential candidates from a functional food Zanthoxyli Pericarpium (Sichuan pepper) for the management of hyperuricemia: high-through virtual screening, network pharmacology and dynamics simulations.Front Endocrinol (Lausanne). 2024 Dec 11;15:1436360. doi: 10.3389/fendo.2024.1436360. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39722812 Free PMC article.
References
-
- Tsai P.W., Chen Y.T., Hsu P.C., Lan C.Y. Study of Candida albicans and Its Interactions with The Host: A Mini Review. Biomed. J. 2013;3:51–64. doi: 10.1016/j.biomed.2012.12.004. - DOI
-
- French L., Horton J., Matousek M. Abnormal Vaginal Discharge: What Does and Does Not Work in Treating Underlying Causes. J. Fam. Pract. 2004;53:890–903. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

