Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation
- PMID: 38067453
- PMCID: PMC10707791
- DOI: 10.3390/molecules28237722
Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation
Abstract
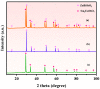
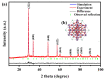
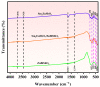
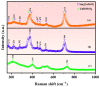
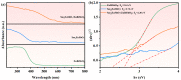
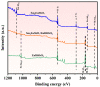
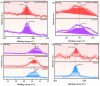
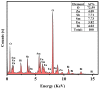
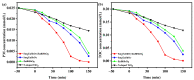
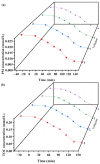
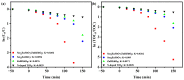
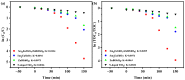
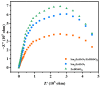
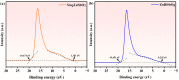
An unprecedented photocatalyst, Sm2EuSbO7, was successfully fabricated in this paper, through a high-temperature solid-state calcination method, which represented its first ever synthesis. Additionally, using the solvothermal method, the Sm2EuSbO7/ZnBiSbO5 heterojunction photocatalyst (SZHP) was fabricated, marking its debut in this study. XRD analysis confirmed that both Sm2EuSbO7 and ZnBiSbO5 exhibited pyrochlore-type crystal structures with a cubic lattice, belonging to the Fd3m space group. The crystal cell parameter was determined to be 10.5682 Å or 10.2943 Å for Sm2EuSbO7 or ZnBiSbO5, respectively. The band gap width measured for Sm2EuSbO7 or ZnBiSbO5 was 2.73 eV or 2.61 eV, respectively. Under visible light irradiation for 150 min (VLTI-150 min), SZHP exhibited remarkable photocatalytic activity, achieving 100% removal of parathion methyl (PM) concentration and 99.45% removal of total organic carbon (TOC) concentration. The kinetic constant (k) for PM degradation and visible light illumination treatment was determined to be 0.0206 min-1, with a similar constant k of 0.0202 min-1 observed for TOC degradation. Remarkably, SZHP exhibited superior PM removal rates compared with Sm2EuSbO7, ZnBiSbO5, or N-doped TiO2 photocatalyst, accompanied by removal rates 1.09 times, 1.20 times, or 2.38 times higher, respectively. Furthermore, the study investigated the oxidizing capability of free radicals through the use of trapping agents. The results showed that hydroxyl radicals had the strongest oxidative capability, followed by superoxide anions and holes. These findings provide a solid scientific foundation for future research and development of efficient heterojunction compound catalysts.
Keywords: Sm2EuSbO7; Sm2EuSbO7/ZnBiSbO5 heterojunction photocatalyst; degradation mechanism; degradation pathway; parathion methyl; photocatalytic activity; visible light irradiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
The Fabrication and Property Characterization of a Ho2YSbO7/Bi2MoO6 Heterojunction Photocatalyst and the Application of the Photodegradation of Diuron under Visible Light Irradiation.Int J Mol Sci. 2024 Apr 17;25(8):4418. doi: 10.3390/ijms25084418. Int J Mol Sci. 2024. PMID: 38674003 Free PMC article.
-
Preparation and Property Characterization of In2YSbO7/BiSnSbO6 Heterojunction Photocatalyst toward Photocatalytic Degradation of Indigo Carmine within Dye Wastewater under Visible-Light Irradiation.Materials (Basel). 2022 Sep 25;15(19):6648. doi: 10.3390/ma15196648. Materials (Basel). 2022. PMID: 36233988 Free PMC article.
-
Synthesis, Performance Measurement of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst and Photocatalytic Degradation of Direct Orange within Dye Wastewater under Visible Light Irradiation.Materials (Basel). 2022 Jun 3;15(11):3986. doi: 10.3390/ma15113986. Materials (Basel). 2022. PMID: 35683292 Free PMC article.
-
Synthesis and Property Examination of Er2FeSbO7/BiTiSbO6 Heterojunction Composite Catalyst and Light-Catalyzed Retrogradation of Enrofloxacin in Pharmaceutical Waste Water under Visible Light Irradiation.Materials (Basel). 2022 Aug 26;15(17):5906. doi: 10.3390/ma15175906. Materials (Basel). 2022. PMID: 36079288 Free PMC article.
-
Photophysical and Photocatalytic Properties of BiSnSbO₆ under Visible Light Irradiation.Materials (Basel). 2018 Mar 26;11(4):491. doi: 10.3390/ma11040491. Materials (Basel). 2018. PMID: 29587420 Free PMC article.
Cited by
-
The Fabrication and Property Characterization of a Ho2YSbO7/Bi2MoO6 Heterojunction Photocatalyst and the Application of the Photodegradation of Diuron under Visible Light Irradiation.Int J Mol Sci. 2024 Apr 17;25(8):4418. doi: 10.3390/ijms25084418. Int J Mol Sci. 2024. PMID: 38674003 Free PMC article.
References
-
- Aulakh M.K., Kaur S., Pal B., Singh S. Morphological influence of ZnO nanostructures and their Cu loaded composites for effective photodegradation of methyl parathion. Solid State Sci. 2020;99:106045. doi: 10.1016/j.solidstatesciences.2019.106045. - DOI
-
- Moctezuma E., Leyva E., Palestino G., de Lasa H. Photocatalytic degradation of methyl parathion: Reaction pathways and intermediate reaction products. J. Photochem. Photobiol. A Chem. 2007;186:71–84. doi: 10.1016/j.jphotochem.2006.07.014. - DOI
-
- Zhao F., Wang L., Li M., Wang M., Liu G., Ping J. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. Trends Anal. Chem. 2023;165:117152. doi: 10.1016/j.trac.2023.117152. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

