Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction
- PMID: 38067598
- PMCID: PMC10708487
- DOI: 10.3390/molecules28237869
Photoinduced Synthesis of Sulfonyl-Containing Phosphorothioates via a Three-Component Reaction
Abstract
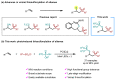
Both sulfonyl and phosphorothioate are important privileged structural motifs which are widely presented in pharmaceuticals and agrochemicals. Herein, we describe an efficient approach to synthesizing sulfonyl-containing phosphorothioates by merging photoredox and copper catalysis at room temperature. This protocol is compatible with a wide range of substrates and can be applied to the late-stage modification of complex molecules. Control experiments are conducted to demonstrate the generation of the sulfonyl radical in the transformation.
Keywords: alkene difunctionalization; copper; multicomponent reaction; phosphorothioate; photoredox catalysis; sulfonyl.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

