Pharmacological Evidence That Dictyostelium Differentiation-Inducing Factor 1 Promotes Glucose Uptake Partly via an Increase in Intracellular cAMP Content in Mouse 3T3-L1 Cells
- PMID: 38067655
- PMCID: PMC10708055
- DOI: 10.3390/molecules28237926
Pharmacological Evidence That Dictyostelium Differentiation-Inducing Factor 1 Promotes Glucose Uptake Partly via an Increase in Intracellular cAMP Content in Mouse 3T3-L1 Cells
Abstract
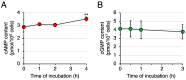
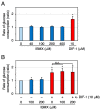
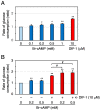
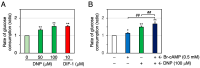
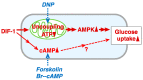
Differentiation-inducing factor 1 (DIF-1) isolated from the cellular slime mold Dictyostelium discoideum can inhibit mammalian calmodulin-dependent cAMP/cGMP phosphodiesterase (PDE1) in vitro. DIF-1 also promotes glucose uptake, at least in part, via a mitochondria- and AMPK-dependent pathway in mouse 3T3-L1 fibroblast cells, but the mechanism underlying this effect has not been fully elucidated. In this study, we investigated the effects of DIF-1 on intracellular cAMP and cGMP levels, as well as the effects that DIF-1 and several compounds that increase cAMP and cGMP levels have on glucose uptake in confluent 3T3-L1 cells. DIF-1 at 20 μM (a concentration that promotes glucose uptake) increased the level of intracellular cAMP by about 20% but did not affect the level of intracellular cGMP. Neither the PDE1 inhibitor 8-methoxymethyl-3-isobutyl-1-methylxanthine at 10-200 μM nor the broad-range PDE inhibitor 3-isobutyl-1-methylxanthine at 40-400 μM had any marked effects on glucose uptake. The membrane-permeable cAMP analog 8-bromo-cAMP at 200-1000 μM significantly promoted glucose uptake (by 20-25%), whereas the membrane-permeable cGMP analog 8-bromo-cGMP at 3-100 μM did not affect glucose uptake. The adenylate cyclase activator forskolin at 1-10 μM promoted glucose uptake by 20-30%. Thus, DIF-1 may promote glucose uptake by 3T3-L1 cells, at least in part, via an increase in intracellular cAMP level.
Keywords: DIF-1; Dictyostelium discoideum; PDE1; cAMP; diabetes; forskolin; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Dictyostelium Differentiation-Inducing Factor 1 Promotes Glucose Uptake via Direct Inhibition of Mitochondrial Malate Dehydrogenase in Mouse 3T3-L1 Cells.Int J Mol Sci. 2024 Feb 4;25(3):1889. doi: 10.3390/ijms25031889. Int J Mol Sci. 2024. PMID: 38339168 Free PMC article.
-
Dictyostelium Differentiation-Inducing Factor-1 Promotes Glucose Uptake, at Least in Part, via an AMPK-Dependent Pathway in Mouse 3T3-L1 Cells.Int J Mol Sci. 2021 Feb 25;22(5):2293. doi: 10.3390/ijms22052293. Int J Mol Sci. 2021. PMID: 33669058 Free PMC article.
-
Dictyostelium differentiation-inducing factor-1 induces glucose transporter 1 translocation and promotes glucose uptake in mammalian cells.FEBS J. 2007 Jul;274(13):3392-404. doi: 10.1111/j.1742-4658.2007.05872.x. Epub 2007 Jun 6. FEBS J. 2007. PMID: 17553062
-
Exploitation of the derivatives of Dictyostelium differentiation-inducing factor-1, which promote glucose consumption in mammalian cells.Life Sci. 2008 Oct 24;83(17-18):608-12. doi: 10.1016/j.lfs.2008.08.012. Epub 2008 Sep 9. Life Sci. 2008. PMID: 18812178
-
Oral administration of Dictyostelium differentiation-inducing factor 1 lowers blood glucose levels in streptozotocin-induced diabetic rats.Life Sci. 2016 Jun 15;155:56-62. doi: 10.1016/j.lfs.2016.04.032. Epub 2016 Apr 27. Life Sci. 2016. PMID: 27131631
Cited by
-
Dictyostelium Differentiation-Inducing Factor 1 Promotes Glucose Uptake via Direct Inhibition of Mitochondrial Malate Dehydrogenase in Mouse 3T3-L1 Cells.Int J Mol Sci. 2024 Feb 4;25(3):1889. doi: 10.3390/ijms25031889. Int J Mol Sci. 2024. PMID: 38339168 Free PMC article.
-
Research on a Minor Organism can also be Benefit the World: The Fascinating Cellular Slime Mold Dictyostelium discoideum.Juntendo Iji Zasshi. 2024 Sep 11;70(5):339-347. doi: 10.14789/jmj.JMJ24-0021-R. eCollection 2024. Juntendo Iji Zasshi. 2024. PMID: 39545231 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

