Evaluation of Femoropopliteal In-Stent Restenosis Characteristics Stratified by Stent Design
- PMID: 38068277
- PMCID: PMC10707093
- DOI: 10.3390/jcm12237225
Evaluation of Femoropopliteal In-Stent Restenosis Characteristics Stratified by Stent Design
Abstract
Purpose: To evaluate the potential differences in characteristics of femoropopliteal in-stent restenosis (ISR) stratified by stent design with a focus on the swirling flow-inducing BioMimics 3D helical centerline stent.
Methods: Patients with ISR of the superficial femoral and popliteal arteries undergoing reintervention were included in this study. The primary endpoint was the angiographic localization and extent of restenosis or reocclusion with the following five different stent systems: SMART Control stent, Supera peripheral stent, GORE® VIABAHN® endoprosthesis, BioMimics 3D stent, and Zilver® PTX® stent.
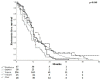
Results: 414 ISR lesions were analyzed, affecting 236 Supera stents, 67 BioMimics 3D stents, 48 Zilver® PTX® stents, 38 SMART Control stents, and 25 VIABAHN® endoprostheses. The mean stent diameter and length were 5.7 ± 0.77 mm and 121.4 ± 94.8 mm, respectively. ISR included 310 (74.9%) lesions with 1 stent, 89 (21.5%) lesions with 2 stents, 14 (3.4%) lesions with 3 stents, and 1 lesion (0.2%) with 4 stents. Most lesions presented as reocclusions (67.4%) rather than focal (13.3%) or diffuse restenoses (19.3%). No significant differences in ISR lesion morphology were found. By trend, BioMimics 3D stent lesion extension was more focal (16.4% versus 12.7%, p = 0.258), with the highest proportion of lesions in which only the proximal stent third was affected (9.0% versus 5.8%, p = 0.230), as compared to the average of the other four devices. The occlusion rate was the second lowest for the BioMimics 3D stent (64.2 vs. 68.0%, p = 0.316). Risk factors for restenosis or occlusion were active smoking, pre-interventional occlusion, and popliteal intervention.
Conclusion: Our results suggest that the helical centerline stent design of the BioMimics 3D stent, which results in a swirling flow with increased wall shear stress, may offer protective properties over straight stent designs, including DES and endoprosthesis, regarding localization and extension of restenosis. Prospective, randomized studies are warranted.
Keywords: endovascular therapy; femoropopliteal artery; in-stent restenosis; peripheral artery disease; stent.
Conflict of interest statement
Elias Noory: Honoraria received from: Bard-BD, Boston Scientific Corp, Abbott Vascular, Medtronic, ShockWave. Thomas Zeller: Honoraria received from: Abbott Vascular, Veryan, Biotronik, Boston Scientific Corp., Cook Medical, Gore & Associates, Medtronic, Philips-Spectranetics, Shockwave. Consulted for: Boston Scientific Corp., Gore & Associates, Medtronic, Veryan, Intact Vascular, Shockwave, Bayer, Vesper Medical. Research, clinical trial, or drug study funds received from (institution): 480 biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Medtronic, Philips, Terumo, TriReme, Shockwave, Med Alliance, Intact Vascular, B. Braun. Common stock: QT Medical. The other authors have no conflict of interest.
Figures
References
-
- Minar E., Pokrajac B., Maca T., Ahmadi R., Fellner C., Mittlbock M., Seitz W., Wolfram R., Potter R. Endovascular brachytherapy for prophylaxis of restenosis after femoropopliteal angio-plasty: Results of a prospective randomized study. Circulation. 2000;102:2694–2699. doi: 10.1161/01.CIR.102.22.2694. - DOI - PubMed
LinkOut - more resources
Full Text Sources

