Effect of Intraperitoneal Chemotherapy with Regorafenib on IL-6 and TNF-α Levels and Peritoneal Cytology: Experimental Study in Rats with Colorectal Peritoneal Carcinomatosis
- PMID: 38068319
- PMCID: PMC10706907
- DOI: 10.3390/jcm12237267
Effect of Intraperitoneal Chemotherapy with Regorafenib on IL-6 and TNF-α Levels and Peritoneal Cytology: Experimental Study in Rats with Colorectal Peritoneal Carcinomatosis
Abstract
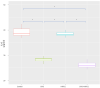
Cytoreductive surgery (CRS), combined with hyperthermic intraperitoneal chemotherapy, has significantly improved survival outcomes in patients with peritoneal carcinomatosis from colorectal cancer (CRC). Regorafenib is an oral agent administered in patients with refractory metastatic CRC. Our aim was to investigate the outcomes of intraperitoneal administration of regorafenib for intraperitoneal chemotherapy (IPEC) or/and CRS in a rat model of colorectal peritoneal metastases regarding immunology and peritoneal cytology. A total of 24 rats were included. Twenty-eight days after carcinogenesis induction, rats were randomized into following groups: group A: control group; group B: CRS only; group C: IPEC only; and group D: CRS + IPEC. On day 56 after carcinogenesis, euthanasia and laparotomy were performed. Serum levels of interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) as well as peritoneal cytology were investigated. Groups B and D had statistically significant lower mean levels of IL-6 and TNF-α compared to groups A and C, but there was no significant difference between them. Both B and D groups presented a statistically significant difference regarding the rate of negative peritoneal cytology, when compared to the control group, but not to group C. In conclusion, regorafenib-based IPEC, combined with CRS, may constitute a promising tool against peritoneal carcinomatosis by altering the tumor microenvironment.
Keywords: colorectal cancer; cytoreductive surgery; intraperitoneal chemotherapy; intraperitoneal injection; peritoneal carcinomatosis; regorafenib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The feasibility and effect of intraperitoneal administration of regorafenib on peritoneal carcinomatosis from colorectal cancer in the rat.Ann Ital Chir. 2022;93:592-598. Ann Ital Chir. 2022. PMID: 36254771
-
[Efficacy of 1 384 cases of peritoneal carcinomatosis underwent cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 Mar 25;24(3):230-239. doi: 10.3760/cma.j.cn.441530-20201110-00603. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 34645167 Chinese.
-
Prospective randomized trial evaluating mandatory second look surgery with HIPEC and CRS vs. standard of care in patients at high risk of developing colorectal peritoneal metastases.Trials. 2010 May 25;11:62. doi: 10.1186/1745-6215-11-62. Trials. 2010. PMID: 20500867 Free PMC article. Clinical Trial.
-
Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases.World J Gastroenterol. 2014 Oct 14;20(38):14018-32. doi: 10.3748/wjg.v20.i38.14018. World J Gastroenterol. 2014. PMID: 25320542 Free PMC article. Review.
-
Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies.Ann Surg. 2006 Feb;243(2):212-22. doi: 10.1097/01.sla.0000197702.46394.16. Ann Surg. 2006. PMID: 16432354 Free PMC article. Review.
References
-
- Sommariva A., Tonello M., Coccolini F., De Manzoni G., Delrio P., Pizzolato E., Gelmini R., Serra F., Rreka E., Pasqual E.M., et al. Colorectal Cancer with Peritoneal Metastases: The Impact of the Results of PROPHYLOCHIP, COLOPEC, and PRODIGE 7 Trials on Peritoneal Disease Management. Cancers. 2022;15:165. doi: 10.3390/cancers15010165. - DOI - PMC - PubMed
-
- Rosa F., Galiandro F., Ricci R., Di Miceli D., Quero G., Fiorillo C., Cina C., Alfieri S. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Colorectal Peritoneal Metastases: Analysis of Short- and Long-Term Outcomes. Langenbeck’s Arch. Surg. 2021;406:2797–2805. doi: 10.1007/s00423-021-02353-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

