Pathomechanisms of Diabetic Kidney Disease
- PMID: 38068400
- PMCID: PMC10707303
- DOI: 10.3390/jcm12237349
Pathomechanisms of Diabetic Kidney Disease
Abstract
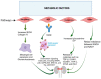
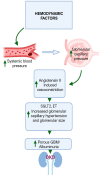
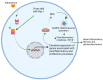
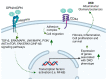
The worldwide occurrence of diabetic kidney disease (DKD) is swiftly rising, primarily attributed to the growing population of individuals affected by type 2 diabetes. This surge has been transformed into a substantial global concern, placing additional strain on healthcare systems already grappling with significant demands. The pathogenesis of DKD is intricate, originating with hyperglycemia, which triggers various mechanisms and pathways: metabolic, hemodynamic, inflammatory, and fibrotic which ultimately lead to renal damage. Within each pathway, several mediators contribute to the development of renal structural and functional changes. Some of these mediators, such as inflammatory cytokines, reactive oxygen species, and transforming growth factor β are shared among the different pathways, leading to significant overlap and interaction between them. While current treatment options for DKD have shown advancement over previous strategies, their effectiveness remains somewhat constrained as patients still experience residual risk of disease progression. Therefore, a comprehensive grasp of the molecular mechanisms underlying the onset and progression of DKD is imperative for the continued creation of novel and groundbreaking therapies for this condition. In this review, we discuss the current achievements in fundamental research, with a particular emphasis on individual factors and recent developments in DKD treatment.
Keywords: hemodynamic; inflammatory and fibrotic factors; metabolic; mineralocorticoid receptor; osteopontin; renin angiotensin aldosterone system; targeted therapies.
Conflict of interest statement
S.B.N. reports grant funding from: NIH/NCATS, NIH/NIMHD, CDC, Travere, Bayer; Editorial Board: American Journal of Kidney Disease; Associate Editor, Journal of the American Society of Nephrology; Advisory Board/Steering Committee: Boehringer Ingelheim/Lilly Pharmaceuticals, AstraZeneca, Bayer, NovoNordisk, Janssen Pharmaceutical; Consultant/National Leader: Bayer-ND-CKD; Honoraria: 2021–2022, 2023 Hypertension Highlights, ADA, 2023 ASN nephSAP. S.K.S. has no conflict of interest.
Figures




References
-
- Tuttle K.R., Bakris G.L., Bilous R.W., Chiang J.L., de Boer I.H., Goldstein-Fuchs J., Hirsch I.B., Kalantar-Zadeh K., Narva A.S., Navaneethan S.D., et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

