From Crafoord's End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions
- PMID: 38068402
- PMCID: PMC10707478
- DOI: 10.3390/jcm12237350
From Crafoord's End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions
Abstract
First described in 1760 by the anatomist Morgagni, coarctation of the aorta (CoA) is a congenital heart defect characterized by narrowing the aorta, typically distal to the left subclavian artery. It accounts for approximately 5-8% of all congenital heart diseases, with an incidence estimated at 4 per 10,000 live births. In 1944, the Swedish surgeon Clarence Crafoord achieved the first successful surgical CoA repair by performing an aortic end-to-end anastomosis on two patients aged 12 and 27 years old. Presently, the most prevalent techniques for surgical repair, particularly in infants and neonates with isolated coarctation, involve resection with end-to-end anastomosis (EEA) and the modified Crafoord technique (extended resection with end-to-end anastomosis (EEEA)). Subclavian flap aortoplasty (SCAP) is an alternative surgical option for CoA repair in patients under two years of age. In cases where the stenosis extends beyond resection and end-to-end anastomosis feasibility, patch aortoplasty (PP) employing a prosthetic patch can augment the stenotic region, especially for older patients. Despite advances in pediatric cardiology and cardiac surgery, recoarctation remains a significant concern after surgical or interventional repair. This comprehensive review aims to provide a thorough analysis of coarctation management, covering the pioneering techniques introduced by Crafoord using end-to-end anastomosis and now extending to the contemporary era marked by percutaneous interventions as well as the recoarctation rate associated with each type.
Keywords: coarctation of the aorta; recoarctation; surgical techniques; transcatheter intervention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


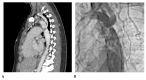

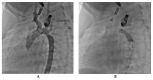



References
-
- Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M., Carnethon M.R., Dai S., De Simone G., Ford E.S., et al. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. - DOI - PMC - PubMed
-
- Crafoord C., Nylin G. Congenital coarctation of the aorta and its surgical treatment. J. Thorac. Surg. 1945;14:347. doi: 10.1016/S0096-5588(20)31801-8. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

