Arabinogalactan-Proteins as Boron-Acting Enzymes, Cross-Linking the Rhamnogalacturonan-II Domains of Pectin
- PMID: 38068557
- PMCID: PMC10707938
- DOI: 10.3390/plants12233921
Arabinogalactan-Proteins as Boron-Acting Enzymes, Cross-Linking the Rhamnogalacturonan-II Domains of Pectin
Abstract
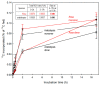
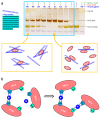
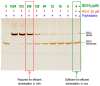
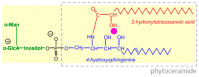
Most pectic rhamnogalacturonan-II (RG-II) domains in plant cell walls are borate-bridged dimers. However, the sub-cellular locations, pH dependence, reversibility and biocatalyst involvement in borate bridging remain uncertain. Experiments discussed here explored these questions, utilising suspension-cultured plant cells. In-vivo pulse radiolabelling showed that most RG-II domains dimerise extremely quickly (<4 min after biosynthesis, thus while still intraprotoplasmic). This tallies with the finding that boron withdrawal causes cell wall weakening within 10-20 min, and supports a previously proposed biological role for boron/RG-II complexes specifically at the wall/membrane interface. We also discuss RG-II monomer ↔ dimer interconversion as monitored in vitro using gel electrophoresis and a novel thin-layer chromatography method to resolve monomers and dimers. Physiologically relevant acidity did not monomerise dimers, thus boron bridge breaking cannot be a wall-loosening mechanism in 'acid growth'; nevertheless, recently discovered RG-II trimers and tetramers are unstable and may thus underpin reversible wall loosening. Dimerising monomers in vitro by B(OH)3 required the simultaneous presence of RG-II-binding 'chaperones': co-ordinately binding metals and/or ionically binding cationic peptides. Natural chaperones of the latter type include highly basic arabinogalactan protein fragments, e.g., KHKRKHKHKRHHH, which catalyse a reaction [2 RG-II + B(OH)3 → RG-II-B-RG-II], suggesting that plants can 'enzymically' metabolise boron.
Keywords: Ca2+; Pb2+; acid-growth; arabinogalactan-proteins; borate diesterase; boron; chaperones; pectin; rhamnogalacturonan-II; trimers of RG-II.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
An Arabidopsis thaliana arabinogalactan-protein (AGP31) and several cationic AGP fragments catalyse the boron bridging of rhamnogalacturonan-II.Biochem J. 2022 Sep 30;479(18):1967-1984. doi: 10.1042/BCJ20220340. Biochem J. 2022. PMID: 36062804 Free PMC article.
-
Making and breaking of boron bridges in the pectic domain rhamnogalacturonan-II at apoplastic pH in vivo and in vitro.Plant J. 2023 Mar;113(6):1310-1329. doi: 10.1111/tpj.16112. Epub 2023 Feb 8. Plant J. 2023. PMID: 36658763 Free PMC article.
-
Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion.Plant J. 2014 Feb;77(4):534-46. doi: 10.1111/tpj.12403. Epub 2014 Jan 24. Plant J. 2014. PMID: 24320597 Free PMC article.
-
Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide.Annu Rev Plant Biol. 2004;55:109-39. doi: 10.1146/annurev.arplant.55.031903.141750. Annu Rev Plant Biol. 2004. PMID: 15377216 Review.
-
Regulation, Diversity and Evolution of Boron Transporters in Plants.Plant Cell Physiol. 2021 Sep 24;62(4):590-599. doi: 10.1093/pcp/pcab025. Plant Cell Physiol. 2021. PMID: 33570563 Review.
Cited by
-
Automated mass spectrometry-based profiling of multi-glycosylated glycosyl inositol phospho ceramides (GIPC) reveals specific series GIPC rearrangements during barley grain development and heat stress response.Plant J. 2025 Jun;122(6):e70279. doi: 10.1111/tpj.70279. Plant J. 2025. PMID: 40570361 Free PMC article.
-
Storming the barricades of rhamnogalacturonan-II synthesis and function.Plant Cell. 2025 Jun 4;37(6):koaf088. doi: 10.1093/plcell/koaf088. Plant Cell. 2025. PMID: 40237333 Free PMC article. Review.
-
Cell wall bricks of defence: the case study of oligogalacturonides.Front Plant Sci. 2025 Mar 25;16:1552926. doi: 10.3389/fpls.2025.1552926. eCollection 2025. Front Plant Sci. 2025. PMID: 40201780 Free PMC article. Review.
-
Fruit softening: evidence for rhamnogalacturonan lyase action in vivo in ripe fruit cell walls.Ann Bot. 2024 Apr 23;133(4):547-558. doi: 10.1093/aob/mcad197. Ann Bot. 2024. PMID: 38180460 Free PMC article.
References
-
- Warington K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923;37:629–672. doi: 10.1093/oxfordjournals.aob.a089871. - DOI
-
- Glime J.M. Nutrient relations: Requirements and sources. In: Glime J.M., editor. Bryophyte Ecology. Volume 1. Michigan Technological University and the International Association of Bryologists; Houghton, MI, USA: 2017. pp. 1–32.
-
- Matsunaga T., Ishii T., Matsumoto S., Higuchi M., Darvill A., Albersheim P., O’Neill M.A. Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol. 2004;134:339–351. doi: 10.1104/pp.103.030072. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

